二乙烯光开关放大光诱导pKa调制
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用分子光开关对酸度进行可逆调节,使光远程控制各种(生物)化学过程成为可能。在此,我们研究了允许放大光诱导的苯酚-二乙烯偶联物的pKa变化的结构特征,该偶联物在光异构化时通过改变可电离部分和吸电子基团之间的偶联在低酸性和高酸性状态之间切换。通过调整这些共轭物的结构,实现了比以前报道的更高的pKa调制幅度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
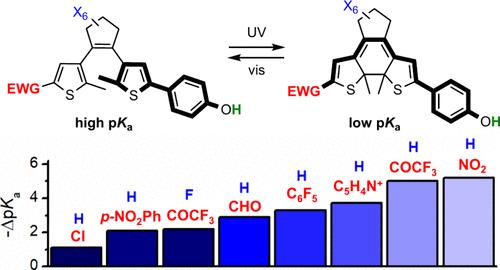
Amplified Light-Induced pKa Modulation with Diarylethene Photoswitches
The reversible modulation of acidity using molecular photoswitches enables the remote control of a variety of (bio)chemical processes with light. Herein we investigated the structural features that allow amplifying photoinduced pKa variation in phenol-diarylethene conjugates, which toggle between low- and high-acidity states by switching the conjugation between the ionizable moiety and electron-withdrawing groups upon photoisomerization. By tuning the structure of these conjugates, high pKa modulation amplitudes were accomplished that surpass those previously reported.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


