egfr突变型非小细胞肺癌治疗前景的变化
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
EGFR突变与EGFR酪氨酸激酶抑制剂(TKIs)疗效之间的关联的发现已经彻底改变了非小细胞肺癌(NSCLC)患者的治疗模式。目前,第三代EGFR TKIs通常以中枢神经系统外显率为特征,是晚期EGFR突变型NSCLC的标准一线治疗方案。第三代EGFR TKIs与抗血管生成药物、化疗、EGFR - met双特异性抗体阿米万他抗或局部肿瘤消融的合理联合正在研究中,作为延迟耐药和增加临床获益的策略。此外,EGFR TKIs正在早期或局部晚期EGFR突变的NSCLC患者中进行评估,其雄心勃勃的目标是实现癌症治愈。尽管获得性耐药是不可避免的挑战,但新兴的治疗方法,如新的TKIs、抗体-药物偶联物、新的免疫治疗方法和靶向蛋白降解剂,在EGFR TKIs治疗或治疗后的EGFR突变NSCLC进展患者中显示出相当大的希望。在这篇综述中,我们描述了目前EGFR突变NSCLC的一线治疗方案,概述了对第三代EGFR TKIs获得性耐药的机制,并探索了新的有希望的治疗策略。我们还强调了未来研究的潜在途径,旨在改善这种疾病患者的生存结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。

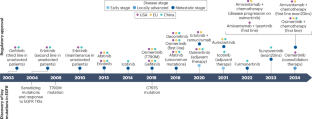
The changing treatment landscape of EGFR-mutant non-small-cell lung cancer
The discovery of the association between EGFR mutations and the efficacy of EGFR tyrosine-kinase inhibitors (TKIs) has revolutionized the treatment paradigm for patients with non-small-cell lung cancer (NSCLC). Currently, third-generation EGFR TKIs, which are often characterized by potent central nervous system penetrance, are the standard-of-care first-line treatment for advanced-stage EGFR-mutant NSCLC. Rational combinations of third-generation EGFR TKIs with anti-angiogenic drugs, chemotherapy, the EGFR–MET bispecific antibody amivantamab or local tumour ablation are being investigated as strategies to delay drug resistance and increase clinical benefit. Furthermore, EGFR TKIs are being evaluated in patients with early stage or locally advanced EGFR-mutant NSCLC, with the ambitious aim of achieving cancer cure. Despite the inevitable challenge of acquired resistance, emerging treatments such as new TKIs, antibody–drug conjugates, new immunotherapeutic approaches and targeted protein degraders have shown considerable promise in patients with progression of EGFR-mutant NSCLC on or after treatment with EGFR TKIs. In this Review, we describe the current first-line treatment options for EGFR-mutant NSCLC, provide an overview of the mechanisms of acquired resistance to third-generation EGFR TKIs and explore novel promising treatment strategies. We also highlight potential avenues for future research that are aimed at improving the survival outcomes of patients with this disease. Third-generation EGFR tyrosine-kinase inhibitors (TKIs) are the standard-of-care first-line treatment for patients with advanced-stage EGFR-mutant NSCLC and their efficacy is being investigated in early stage and locally advanced disease. The authors of this Review describe the current first-line treatment options for EGFR-mutant NSCLC, discuss mechanisms of acquired resistance to third-generation EGFR TKIs and new promising treatment strategies, such as bispecific antibodies, next-generation TKIs, antibody–drug conjugates, immunotherapy approaches and targeted protein degraders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


