人线粒体MRS2通道的结构和功能。
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人类线粒体RNA剪接2蛋白(MRS2)与Mg2+在线粒体内膜上的转运有关,因此在Mg2+稳态中起着重要作用,这对线粒体的完整性和功能至关重要。然而,其基本通道特性(如离子选择性和调控)的分子机制尚不清楚。在这里,我们对MRS2的结构和功能进行了研究。不同离子条件下的低温电子显微镜结构揭示了五聚体通道结构和离子渗透的分子基础和电位调节机制。电生理分析表明,MRS2是一个Ca2+调控的非选择性通道,可渗透到Mg2+、Ca2+、Na+和K+,这与它的原核同源物CorA形成对比,CorA是一个Mg2+门控的Mg2+通道。此外,MRS2孔内保守的精氨酸环限制阳离子运动,从而防止通道破坏驱动线粒体三磷酸腺苷合成的质子动力。总之,我们的研究结果为进一步了解MRS2在线粒体功能和疾病中的作用提供了一个分子框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。

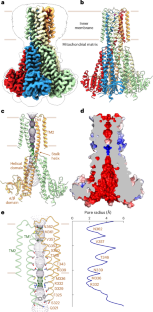
Structure and function of the human mitochondrial MRS2 channel
The human mitochondrial RNA splicing 2 protein (MRS2) has been implicated in Mg2+ transport across mitochondrial inner membranes, thus having an important role in Mg2+ homeostasis critical for mitochondrial integrity and function. However, the molecular mechanisms underlying its fundamental channel properties such as ion selectivity and regulation remain unclear. Here we present a structural and functional investigation of MRS2. Cryo-electron microscopy structures in various ionic conditions reveal a pentameric channel architecture and the molecular basis of ion permeation and potential regulation mechanisms. Electrophysiological analyses demonstrate that MRS2 is a Ca2+-regulated, nonselective channel permeable to Mg2+, Ca2+, Na+ and K+, which contrasts with its prokaryotic ortholog, CorA, operating as a Mg2+-gated Mg2+ channel. Moreover, a conserved arginine ring within the pore of MRS2 functions to restrict cation movements, thus preventing the channel from collapsing the proton motive force that drives mitochondrial adenosine triphosphate synthesis. Together, our results provide a molecular framework for further understanding MRS2 in mitochondrial function and disease. Using structural and electrophysiological analysis, authors demonstrated that the mitochondrial MRS2 channel is a calcium-regulated nonselective cation channel.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


