栀子花蕾香豆素衍生物的结构多样性及其细胞毒活性。
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从栀子花干芽中分离鉴定出5种新的香豆素,包括3种苯丙化香豆素A-C(1-3), 2种双香豆素D和E(4和5),以及8种已知的类似物。通过对它们的NMR、IR、UV、HR-ESI-MS和ECD数据的综合分析,对它们的结构进行了鉴定。此外,经酸性水解和化学衍生化后,通过HPLC分析确定了葡萄糖片段的绝对构型。测定了各化合物对A549和SMMC-7721细胞株的细胞毒活性。其中化合物12对两株细胞系的IC50值分别为22.65 μM和25.83 μM,毒性最强。与顺铂(29.78 μM)相比,化合物12对A549细胞株具有更强的细胞毒活性。初步的构效关系表明,香豆素骨架上的7-羟基在结构上是对A-549和SMMC-7721细胞株具有细胞毒性所必需的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
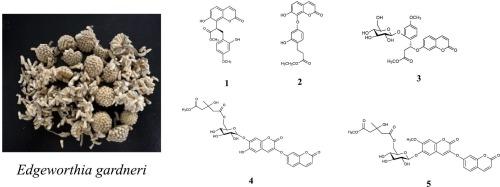
Structurally diverse coumarin derivatives from the buds of Edgeworthia gardneri and their cytotoxic activities
Five new coumarins, including three phenypropaninized coumarins edgegardnerols A–C (1–3), two bicoumarins edgegardnerols D and E (4 and 5), along with eight known analogues, were isolated and identified from the dried buds of E. gardneri. Their structures were elucidated by comprehensive analysis of their NMR, IR, UV, HR-ESI-MS, and ECD data. Furthermore, the absolute configurations of the glucose moiety were confirmed by HPLC analysis after acidic hydrolysis and chemical derivatization. The cytotoxic activities of all isolated compounds were evaluated against A549 and SMMC-7721 cell lines. Among the tested isolates, compound 12 showed the most potent cytotoxicity with IC50 values of 22.65 μM and 25.83 μM in these two cell lines, respectively. Compared with cisplatin (29.78 μM), compound 12 exhibited stronger cytotoxic activity in A549 cell line. The preliminary structure-activity relationship showed that 7-hydroxy group on coumarin skeleton was structurally required for the cytotoxity against the A-549 and SMMC-7721 cell lines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


