揭示苯胺作为Brønsted碱在rh催化的C−H烷基化中的作用:对氨基辅助外球CMD的计算见解
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在Rh催化苯胺衍生物烷基化反应中,C−H键的裂解是形成新的C−Rh键的关键步骤。由于苯胺固有的碱性,在这些类型的反应中,它是否能够作为Brønsted碱来辅助C−H键的裂解一直存在争议。在本报告中,我们提出了支持氨基辅助外球协同金属-去质子化(CMD)机制的计算证据。在这里,苯胺作为Brønsted碱,促进C−H键的切割,而Rh和苯胺之间没有任何直接的相互作用。与内球CMD和其他碱基辅助的C - H键裂解途径相比,这种氨基辅助的外球CMD途径能量更低,为rh催化苯胺衍生物C - H功能化的机理提供了新的见解。几何分析阐明了C−H键解理过程的细节。在Rh催化苯胺衍生物与烯丙醇氧化C - H烷基化过程中C - H键裂解机理的研究是氨基辅助外球CMD参与Rh催化的一个重要案例。我们预计,新的氨基辅助外球CMD模式可以扩展到其他C−H键功能化过程,并解释苯胺在许多情况下的独特有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
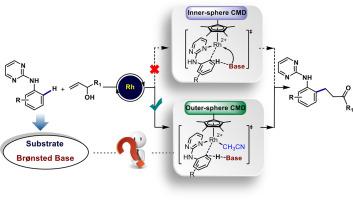
Unveiling the role of aniline as a Brønsted base in Rh-catalyzed C−H alkylation: Computational insights into amino-assisted outer-sphere CMD
C−H bond cleavage is a key step for the formation of new C−Rh bond in Rh-catalyzed alkylation of aniline derivatives. Due to the inherent basicity of aniline, there is ongoing debate regarding its ability to act as a Brønsted base to assist C−H bond cleavage in these type reactions. In this report, we present computational evidence supporting an amino-assisted outer-sphere concerted metalation-deprotonation (CMD) mechanism. Here, the aniline acts as a Brønsted base to facilitate C−H bond cleavage without any direct interaction between the Rh and the aniline. This amino-assisted outer-sphere CMD pathway is found to be lower in energy compared with the inner-sphere CMD and other base-assisted C−H bond cleavage pathways, providing new insights into the mechanism of Rh-catalyzed C−H functionalization of aniline derivatives. Geometric analysis was conducted to elucidate details of the C−H bond cleavage process. This mechanistic study of C−H bond cleavage in Rh-catalyzed oxidative C−H alkylation of aniline derivatives with allyl alcohols is an important case study of the involvement of amino-assisted outer-sphere CMD in Rh catalysis. We anticipate that the novel amino-assisted outer-sphere CMD mode may extend to other C−H bond functionalization processes and explain the unique effectiveness of aniline in many instances.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


