c型凝集素9参与木栗的免疫应答、发育和繁殖
IF 4.2
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
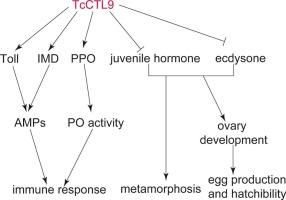
c型凝集素(ctl)作为一大家族的模式识别受体(PRRs),已被报道参与细菌感染,但ctl在昆虫发育中的作用尚不清楚。Tribolium castaneum CTL9 (TcCTL9)同源物在昆虫中已被鉴定,但其功能尚不清楚。因此,我们在本研究中对TcCTL9进行了功能分析。我们的研究结果表明,TcCTL9可以通过脂多糖和肽聚糖与细菌结合,并以Ca2+依赖的方式凝集革兰氏阳性和革兰氏阳性细菌。沉默TcCTL9降低了对金黄色葡萄球菌和大肠杆菌的免疫抗性,降低了抗菌肽和酚氧化酶原的表达,抑制了酚氧化酶活性。这些数据表明,TcCTL9通过Toll和IMD途径以及酚氧化酶原系统在免疫应答中起作用。在发育过程中,TcCTL9在卵-幼虫和蛹-成虫阶段均有高表达,敲低TcCTL9可通过蜕皮激素和幼体激素途径抑制板栗虫的蜕变、产卵和孵化以及卵巢发育。本研究全面阐明了TcCTL9同源物在昆虫中的功能,为开发新的农药靶点提供了理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
C-type lectin 9 participates in the immune response, development and reproduction of Tribolium castaneum
C-type lectins (CTLs), as a large family of pattern recognition receptors (PRRs), have been reported to be involved in bacterial infection, but the role of CTLs in development has been poorly understood in insects. The orthologues of Tribolium castaneum CTL9 (TcCTL9) have been identified among insects, but its functions were currently unclear. Therefore, we performed functional analysis of TcCTL9 in this study. Our results indicated that TcCTL9 could bind to bacteria through lipopolysaccharide and peptidoglycan, and agglutinate Gram-positive and Gram-positive bacteria in a Ca2+-dependent manner. Silencing TcCTL9 reduced the immune resistance to Staphylococcus aureus and Escherichia coli, decreased the expression of antimicrobial peptides and prophenoloxidase, and inhibited the phenoloxidase activity. These data suggested that TcCTL9 functioned in the immune response via the Toll and IMD pathways and prophenoloxidase system. During development, TcCTL9 had high expression in the periods of egg to larva and pupa to adult, and knockdown of TcCTL9 suppressed the metamorphosis, egg production and hatchability, and ovary development through ecdysone and juvenile hormone pathways in T. castaneum. This study comprehensively clarified the functions of TcCTL9 orthologues in insects and provided the theoretical basis for developing novel targets of pesticides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


