钯包覆富勒烯(TM-Pd@C60)修饰的过渡金属单碳化物(TM: CrC, FeC, MnC, TiC, VC)在析氢反应(HER)中的电催化性能
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
为析氢反应(HER)寻找高效、经济的电催化剂对于推进可再生能源技术至关重要。本研究采用密度泛函理论(DFT)在MN12-SX/GenECP/Def2svp/LanL2DZ计算方法研究了包覆钯(Pd)并掺杂过渡金属碳化物(CrC、FeC、MnC、TiC和VC)的改性富勒烯(C60)体系的电子、结构和催化性能。前沿分子轨道(FMO)分析显示,掺杂后HOMO-LUMO间隙发生了显著变化,表明电子导电性的增强对催化活性至关重要。MnC -Pd@C60的最小能隙为0.081 eV, VC-Pd@C60的最小能隙为0.096 eV,显示了它们在电子转移方面的就绪性。通过计算吸附氢的吉布斯自由能变化(ΔGH)来评估HER活性。结果表明TiC - Pd@C60, FeC - Pd@C60和CrC - Pd@C60的最佳ΔGH值接近于零,表明它们具有优越的催化性能。结构分析证实了这些掺杂体系的稳定性,在金属封装和掺杂后,在富勒烯框架中观察到最小的畸变。振动分析表明,Pd@C₆₀配合物的M - C振动减少,M - H键的形成表明其对氢的吸附效果较好,特别是在MnC -Pd@C和VC-Pd@C₆₀中。热力学研究表明,MnC -Pd@C₆0和VC-Pd@C₆0表现出高度的放热自发氢吸附。总的来说,Ti、Fe和cr基催化剂表现出较弱的相互作用,这可能有利于HER中的解吸步骤。另一方面,含有V和Mn的催化剂通过Tafel步骤表现出强烈的氢相互作用,这可能有利于初始氢吸附,但可能需要优化以确保有效的氢释放。本文章由计算机程序翻译,如有差异,请以英文原文为准。
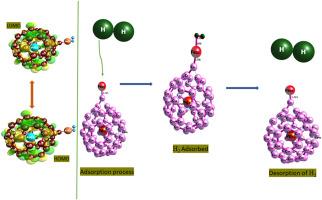
Electrocatalytic performance of transition metal mono-carbides (TM: CrC, FeC, MnC, TiC, VC) decorated on palladium-encapsulated fullerenes (TM-Pd@C60) for hydrogen evolution reaction (HER): A DFT perspective
The search for efficient and cost-effective electrocatalysts for the hydrogen evolution reaction (HER) is critical for advancing renewable energy technologies. This study employs density functional theory (DFT) at the MN12-SX/GenECP/Def2svp/LanL2DZ computational method to investigate the electronic, structural, and catalytic properties of modified fullerene (C60) systems encapsulated with palladium (Pd) and doped with transition metal carbides: CrC, FeC, MnC, TiC, and VC. Frontier molecular orbital (FMO) analysis reveals significant changes in the HOMO-LUMO gap upon doping, indicating enhanced electronic conductivity essential for catalytic activity. The lowest energy gaps 0.081 eV for MnC–Pd@C60 and 0.096 eV for VC-Pd@C60 were observed thus showing their readiness in terms of electron transfer. HER activity was assessed through the calculation of Gibbs free energy changes (ΔGH) for hydrogen adsorption. The results highlight TiC–Pd@C60, FeC–Pd@C60, and CrC–Pd@C60 as having optimal ΔGH values close to zero, suggesting their superior catalytic performance. Structural analysis confirms the stability of these doped systems, with minimal distortions observed in the fullerene framework upon metal encapsulation and doping. Vibrational analysis revealed that Pd@C₆₀ complexes show reduced M − C vibrations and the formation of M − H bonds, indicating efficient hydrogen adsorption, especially in MnC–Pd@C₆₀ and VC-Pd@C₆₀. Thermodynamics investigation show MnC–Pd@C₆₀ and VC-Pd@C₆₀ to exhibit highly exothermic and spontaneous hydrogen adsorption. Overall, Ti, Fe, and Cr-based catalysts show weaker interactions, which might favor the desorption step in HER. On the other hand, catalysts with V and Mn show strong hydrogen interactions via the Tafel step, which might benefit initial hydrogen adsorption but could require optimization to ensure efficient hydrogen release.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


