氯胆碱型深共晶溶剂在水体系中抑制甲烷水合物形成的相平衡及动力学研究
引用次数: 0
摘要
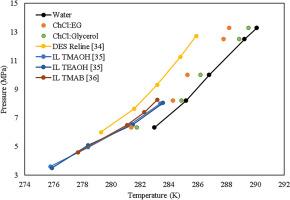
海底管道中的天然气水合物可能会导致堵塞,并可能导致爆炸,而深共晶溶剂(DESs)是传统化学抑制剂的替代选择,或者在与其他化学品混合时可以最大限度地减少其使用。采用微差扫描量热法(μ-DSC)研究了氯化胆碱(ChCl)与甘油和乙二醇溶液的热力学水合物抑制(THI)和动力学水合物抑制(KHI)行为。将制备好的DES在水中稀释,制备了DES-in-water体系。水- des和des -水中系统的区别是基于稀释程度。对于DES-in-water系统,水中浓度较高,DES成分较少。然而,水-DES系统涉及到向DES中加入少量水,这可能会破坏DES内的氢键网络,导致其物理和化学性质的变化。DES溶液的浓度为10%和15%,压力范围为6.32 ~ 13.27 MPa,水-液-气平衡温度范围为281.4 ~ 290.1 k。这两种化合物都是甲烷天然气水合物的热力学和动力学水合物抑制剂。计算了五个压力值的HLVE。THI结果表明,ChCl:乙二醇的平均降温(ADT)为1.47 K,高于ChCl:甘油在10 wt%时的平均降温(ADT)为0.50 K。此外,在动力学水合物抑制方面,ChCl:乙二醇的性能优于ChCl:甘油。ChCl:乙二醇在14.1 bar下的最高诱导时间为1.5 h,而ChCl:甘油在相同压力下的最高诱导时间为1.2 h。采用Dickens和Quinby-Hunt模型对甲烷水合物进行了热力学水合物建模。总体平均绝对误差(MAE)值为0.26 K,而ChCl:乙二醇体系的MAE值为0.32 K。两个系统的R2值均大于0.90,证明模型拟合良好。由于其对环境无害的特性,DESs具有在实际流动保障应用中应用的潜力。这项工作是新颖的,因为它研究了DESs在高压下对甲烷水合物的抑制作用以及热力学建模。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Phase equilibrium and kinetic studies of choline chloride-based deep eutectic solvents in water system for the inhibition of methane gas hydrate formation
Gas hydrates in subsea pipelines can lead to blockages, potentially causing explosions, and Deep Eutectic Solvents (DESs) offer an alternative to traditional chemical inhibitors or can minimize their usage when mixed with other chemicals. The thermodynamic hydrate inhibition (THI) and kinetic hydrate inhibition (KHI) behavior of two DESs i.e., choline chloride (ChCl) solution with glycerol and ethylene glycol are investigated using Micro Differential Scanning Calorimetry (μ-DSC). The DES-in-water systems were prepared by diluting the prepared DES in water. The difference between water-in-DES and DES-in-water systems is based on the extent of dilution. For DES-in-water systems, the water is in higher concentration and DES is a minor component. Whereas, water-in-DES systems involve adding a small amount of water to a DES. This can disrupt the hydrogen bonding network within the DES, leading to changes in its physical and chemical properties. The concentration of the DES solution was 10 and 15 wt% and the study was performed in-between the pressure range of 6.32–13.27 MPa while the Hydrate-Liquid-Vapor-Equilibrium (HLVE) temperature lies between the range of 281.4–290 .1K. Both compounds acted as thermodynamic and kinetic hydrate inhibitors for methane gas hydrates. HLVE was calculated for five pressure values. THI results show that the average depression temperature (ADT) of ChCl: Ethylene glycol is 1.47 K which is higher than the ADT achieved by ChCl: glycerol of 0.50 K at 10 wt%. Also, regarding kinetic hydrate inhibition, ChCl: Ethylene glycol showed better performance than ChCl: glycerol. The highest induction time attained by ChCl: Ethylene glycol is 1.5 h at 14.1 bar while for ChCl: glycerol, it is 1.2 h at the same pressure. Thermodynamic hydrate modeling for methane hydrates was also performed using the Dickens and Quinby-Hunt model. It showed an overall Mean Absolute error (MAE) value of 0.26 K while for the ChCl: Ethylene Glycol system, the MAE value is 0.32 K. The R2 value was higher than 0.90 for both systems, proving the model's good fit. DESs have the potential to be applied in practical flow assurance applications due to their environmentally benign properties. The work is novel as it investigates the use of DESs for methane hydrate inhibition at high pressure along with the thermodynamic modeling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


