透明质酸引起炎症,但抑制巨噬细胞的吞噬功能
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
细胞外基质(ECM)为心肌提供结构和功能支持,但心肌梗死(MI)改变了细胞外基质的组成。ECM的主要成分之一透明质酸(HA)在心肌梗死后积累;然而,ha的特异性生物学作用——特别是在浸润免疫细胞水平上的作用以及这种相互作用对心室重塑的影响——尚未被探索。由于心肌梗死后HA的急性积累与巨噬细胞浸润一致,我们评估了HA对巨噬细胞功能的影响。结果与假手术心脏相比,HA水平在梗死心脏和梗死心脏的远端区域均升高。由于心肌梗死后HA的急性积累与巨噬细胞浸润相吻合,我们探讨了HA积累对巨噬细胞功能各个终点的影响,包括巨噬细胞活化、吞噬和efferocytosis。我们的数据表明,巨噬细胞暴露于HAHMW会促使巨噬细胞向更亲炎的表型发展,如促炎信号如IL-2、IL-17和IP-10的分泌增加。我们的数据还表明,在HA存在的情况下,巨噬细胞efferocytosis和fc受体依赖性吞噬都受到抑制。这些结果是HAHMW治疗所特有的,因为当细胞用HALMW治疗时没有观察到类似的结果。利用来自Cd44 - / -小鼠的巨噬细胞,我们确定,虽然HAHMW对细胞因子分泌的影响似乎部分依赖于Cd44表达,但对巨噬细胞吞噬的影响是独立的。由于心肌梗死后死亡的心肌细胞和细胞碎片的巨噬细胞efferocysis是至关重要的,我们认为这种反应将延长炎症的消退并导致适应性不良的重塑。结论血凝素在心肌梗死后积累,可能促进巨噬细胞的促炎表型。未来的研究将探索梗死后HA积累在体内调节心脏巨噬细胞动力学和功能的程度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
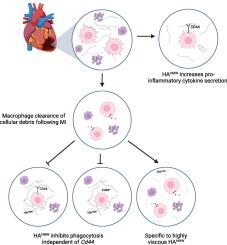
Hyaluronan provokes inflammation but suppresses phagocytotic function in macrophages
Background
The extracellular matrix (ECM) provides structural and functional support for the myocardium, but myocardial infarction (MI) changes the composition of the ECM. One of the chief components of the ECM, hyaluronan (HA), accumulates after MI; however, specific biological actions of HA—particularly at the level of infiltrating immune cells and implications of such interactions on ventricular remodeling—have not been explored.
Goal
Because acute accumulation of HA coincides with macrophage infiltration after MI, we assessed the impact of HA on macrophage function.
Results
Compared to SHAM hearts, HA levels were elevated in both the infarct and remote regions of infarcted hearts. Because acute accumulation of HA coincides with macrophage infiltration after MI, we explored the implication of HA accumulation on various endpoints of macrophage function, including macrophage activation, phagocytosis, and efferocytosis. Our data suggests that exposing macrophages to HAHMW pushes macrophages toward a more pro-inflammatory phenotype as indicated by increased secretion of pro-inflammatory signals such as IL-2, IL-17, and IP-10. Our data also suggests that in the presence of HA, both macrophage efferocytosis and Fc-receptor dependent phagocytosis are suppressed. These results are unique to treatment with HAHMW, as similar results were not observed when cells were treated with HALMW. Using macrophages from Cd44−/− mice, we determined that while the impact of HAHMW on cytokine secretion does seem to be dependent in part on Cd44 expression, the impact on macrophage phagocytosis is independent. Since macrophage efferocytosis of dying cardiomyocytes and cellular debris is critical following MI, we believe that this response will prolong the resolution of inflammation and lead to maladaptive remodeling.
Conclusion
HA accumulates post-MI and may promote a pro-inflammatory phenotype in macrophages. Future studies will explore the extent to which post infarct HA accumulation regulates cardiac macrophage dynamics and function in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


