基于载铁蛋白的自组装纳米疫苗:开发和疫苗方案评价
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-28
DOI:10.1016/j.ejpb.2024.114589
引用次数: 0
摘要
载铁蛋白为基础的系统已经探索了过去十年的潜力,作为疫苗输送的病毒性疾病。基于载铁蛋白的纳米级系统可以提高免疫原性,有助于抗原稳定性,并减少疫苗的不良反应。表皮生长因子受体(EGFRvIII肽,PEPvIII)的细胞外突变部分由于在正常细胞中很少表达,可以作为特异性肿瘤抗原。在此背景下,本研究提出了一种基于载铁蛋白的系统(AFt)的开发和免疫原性评估,以提供具有抗肿瘤目的的肽疫苗。我们开发了一种具有不同PEPvIII:AFt比例的配方,并在关联效率分析中确定了配方中AFt浓度与PEPvIII关联百分比之间的相关性。此外,由于不同的关联效率,观察到针对EGFRvIII的免疫应答差异取决于PEPvIII浓度。最后,体液免疫反应结果显示,针对AFt的抗体产生量高,这可能影响免疫耐受。总的来说,本研究建立了PEPvIII:AFt的配方流程,突出了保证疫苗安全性和有效性的决定因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
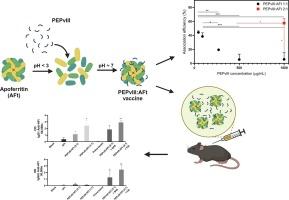
Self-assembled nanovaccine based on apoferritin: Development and vaccine regimen evaluation
Apoferritin-based systems have been explored last decade for their potential as vaccine delivery for viral diseases. The nanosized properties of an apoferritin-based system could increase immunogenicity, contribute to antigen stability, and reduce the vaccines’ adverse effects. The mutated extracellular portion of the epidermal growth factor receptor (EGFRvIII peptide, PEPvIII) can be applied as a specific tumoral antigen due to rare expression in normal cells. In this context, the present study proposed the development and the immunogenicity evaluation of an apoferritin-based system (AFt) to deliver a peptide vaccine for an antitumoral purpose. We developed a formulation with different PEPvIII:AFt ratios and during the association efficiency analysis, identified the dependence between the AFt concentration and the PEPvIII association percentage in the formulation. Besides, differences in the immune responses against EGFRvIII were observed depending on the PEPvIII concentration due to the different association efficiencies. Finally, the humoral immune response results showed a high antibody production against AFt, which might affect the immunological tolerance. Collectively, this study establishes the PEPvIII:AFt formulation process and highlights the determinant factors for guaranteeing vaccine safety and efficacy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


