用中间自由基分析解决DBD等离子体辅助燃烧下NH3/H2双燃料火焰NOX形成机理
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
为了实现碳中和,采用含氨(NH3)/氢(H2)的零碳燃料在实践中越来越受欢迎。本文重点研究了介质阻挡放电(DBD)等离子体辅助NH3/H2双燃料火焰在不同等离子体电压(VAC)和氢比(ZH2)下的燃烧性能和氮氧化物(NOX)形成机制,并利用PLIF和化学发光技术同时解决了这一问题。得到的分析结果表明,VAC对NOX排放有积极的促进作用,其触发NOX形成的阈值为VAC = 11 kV。随着VAC从11 kV不断增加到12.5 kV, NOX急剧增长8.3% ~ 12.3% (ZH2从0.2增加到0.3)。这种现象主要是由于OH自由基的生长传播速度快于NH2自由基。此外,还确定了低生成区、中生成区和高生成区三个NOX生成区域,本质上反映了氢气比对NOX生成的影响大于放电电压。此外,火焰表面密度(FSD,暴露燃烧强度)与NOX之间的相互关系也得到了全面的探讨。高DBD-VAC(低配氢比)显著促进了FSD,燃烧强度较好,但NOX生成不明显。在未来的应用中,可以利用DBD等离子体的高电压来取代氨氢燃烧中的高掺氢比,以获得有效控制NOX的促进燃烧强度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
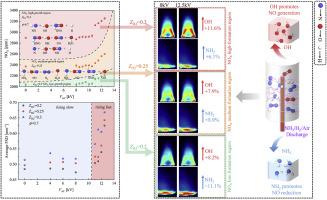
Addressing NOX formation mechanisms of NH3/H2 dual-fuel flame under DBD plasma-assisted combustion resolved by intermediate radicals analysis
In order to attain carbon-neutrality, the implementation of zero-carbon fuel containing ammonia (NH3)/hydrogen (H2) has become more and more practically popular. This work focuses on addressing the combustion performance and nitrogen oxide (NOX) formation mechanisms of dielectric barrier discharge (DBD) plasma-assisted NH3/H2 dual-fuel flames at varied plasma voltages (VAC) and hydrogen ratios (ZH2), which were resolved by PLIF and chemiluminescence techniques concurrently. The analytical results obtained show that VAC had positive effectiveness on contributing to NOX emissions with a threshold of VAC = 11 kV found for triggering NOX formation. With VAC increased from 11 kV to 12.5 kV constantly, NOX grew dramatically by 8.3%–12.3% (ZH2 elevated from 0.2 to 0.3). This phenomenon was mainly because of the growing propagation of OH radicals being faster than that of NH2 radicals. Besides, three NOX formation regions, including low-formation region, medium-formation region and high-formation region were determined, which essentially reflected that hydrogen ratio predominated over discharge voltage on forming NOx. Moreover, the inter-relationship between flame surface density (FSD, revealing combustion intensity) and NOX has been comprehensively explored. And high DBD-VAC (with low hydrogen blending ratio) was found to significantly promote the FSD resulting in better combustion intensity, but caused inappreciable NOX formation. In upcoming future application, high voltage of DBD plasma could be utilized for replacing high hydrogen blending ratio in ammonia/hydrogen combustion, to obtain promotional combustion intensity with effective NOX control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


