利用原子电负性巧妙调节新型苯并唑-4-喹诺酮类荧光团的ESIPT机制
IF 2
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
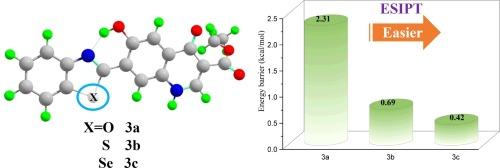
从理论上研究了原子电负性(O, S和Se)对荧光苯并唑基-4-喹诺酮类衍生物激发态分子内质子转移(ESIPT)行为的影响。结构参数和红外振动谱分析表明,随着原子电负性减小(O→S→Se),分子内氢键(O1H1⋯N1)在第一(S1)激发态逐渐加强。拓扑参数、减少密度梯度(RDG)散点图和相互作用区域指示(IRI)等值面进一步证实了我们的结果。分子轨道的能隙反映了原子电负性越小,激发态反应性越强。此外,构建的势能曲线(PECs)显示,Se取代基具有较低的势垒(0.42 kcal/mol),更有可能加速ESIPT过程的发生。这些结果表明原子的电负性有助于调控ESIPT过程,这将为未来设计和合成基于ESIPT的荧光团铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tactfully regulating the ESIPT mechanism of novel benzazolyl-4-quinolones fluorophore by atomic electronegativity
The effects of atomic electronegativity (O, S and Se) on the excited state intramolecular proton transfer (ESIPT) behavior of fluorescent benzazolyl-4-quinolones derivatives have been investigated theoretically. Analysis of structure parameters and infrared vibrational spectra indicate that the intramolecular hydrogen bonds (O1![]() H1⋯N1) are gradually strengthened in the first (S1) excited state as the atomic electronegativity diminishes (O → S → Se). The topological parameters, reduced density gradient (RDG) scatter plots and interaction region indicator (IRI) isosurface further confirm our results. The energy gap of molecular orbitals reflect that the less atomic electronegativity prompt greater excited state reactivity. In addition, the constructed potential energy curves (PECs) reveal that Se substituent has lower potential barrier (0.42 kcal/mol), which is more likely to accelerate the occurrence of ESIPT process. These results show that the atomic electronegativity helps to regulate the ESIPT process, which will pave the way for the design and synthesis of ESIPT-based fluorophores in future.
H1⋯N1) are gradually strengthened in the first (S1) excited state as the atomic electronegativity diminishes (O → S → Se). The topological parameters, reduced density gradient (RDG) scatter plots and interaction region indicator (IRI) isosurface further confirm our results. The energy gap of molecular orbitals reflect that the less atomic electronegativity prompt greater excited state reactivity. In addition, the constructed potential energy curves (PECs) reveal that Se substituent has lower potential barrier (0.42 kcal/mol), which is more likely to accelerate the occurrence of ESIPT process. These results show that the atomic electronegativity helps to regulate the ESIPT process, which will pave the way for the design and synthesis of ESIPT-based fluorophores in future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


