废LiFePO4的FeCl3浸出-喷雾热解法增值回收
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
传统的废磷酸铁锂(LiFePO4, LFP)回收技术通常存在程序复杂、化学品消耗大、经济可行性有限等问题。本文提出了一种将FeCl3浸出与喷雾热解相结合的废LFP高附加值回收方法。以FeCl3为浸出剂,30 min内可选择性浸出99%以上的锂,料液比可达300 g L−1。所得的浸出渣(FePO4)可作为合成新LFP的前体。随后,含Li和fe的渗滤液进行喷雾热解过程,得到由LiCl和Fe2O3组成的热解粉。通过水浸过程,基于LiCl和Fe2O3在水中的独特溶解度,实现了它们的分离。所得的高纯度LiCl溶液和Fe2O3残渣分别可直接用于生产电池级Li2CO3和LFP阴极。使用回收的Li2CO3和FePO4前驱体合成的LFP/C阴极,在1C速率下循环200次后显示出令人印象深刻的99.8%的容量保持率。这种方法在生产高附加值产品的同时避免了酸或碱的消耗,具有很大的经济效益和环境效益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
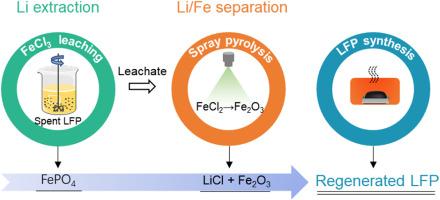
Value-added recycling of spent LiFePO4 by a FeCl3 leaching-spray pyrolysis approach
Traditional recycling technologies for spent lithium iron phosphate (LiFePO4, LFP) usually suffer from issues such as complex procedures, heavy chemical consumption, and limited economic viability. Herein, we propose a method combining FeCl3 leaching and spray pyrolysis for the high-value-added recycling of spent LFP. Using FeCl3 as leaching agent, over 99 % of Li is selectively leached out within 30 min with a solid-liquid ratio up to 300 g L−1. The resulting leaching residue (FePO4) serves as a precursor for the synthesis of new LFP. Subsequently, the Li and Fe-containing leachate undergoes a spray pyrolysis process, yielding a pyrolysis powder comprising LiCl and Fe2O3. Through a water-leaching procedure, the separation of LiCl and Fe2O3 is achieved based on their distinct solubility in water. The resulting high-purity LiCl solution and Fe2O3 residue are directly utilizable for producing battery-grade Li2CO3 and LFP cathodes, respectively. LFP/C cathodes, synthesized using the recovered Li2CO3 and FePO4 precursor, exhibit an impressive 99.8 % capacity retention after 200 cycles at a 1C rate. This method holds great economic and environmental benefits by producing high-value-added products while avoiding the consumption of acids or alkalis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


