聚吡咯改性污泥生物炭去除地下水中铬(VI)的机理研究
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
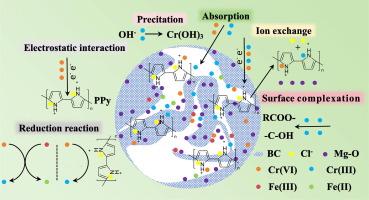
以污泥生物炭为原料,分别以铁和镁为金属源,采用吡咯单体原位氧化聚合法制备了聚吡咯镁改性生物炭(PPy/MBC)和聚吡咯纳米零价铁改性生物炭(PPy/nZVI/BC)两种复合材料。研究了复合材料对地下水中六价铬(Cr(VI))的去除效果及机理。研究结果表明,PPy/MBC的去除效果优于PPy/nZVI/BC。Cr(VI)和Cr(Total)的最高去除率分别为170和120 mg·g−1。吸附动力学服从准二级动力学,吸附等温符合Langmuir吸附等温模型。Elovich模型表明,PPy/MBC对Cr(VI)的吸附是以化学吸附为主的非均相扩散吸附。热力学分析表明,PPy/MBC吸附Cr(VI)为自发吸热反应。改性后的PPy/MBC比表面积达到26.0824 m2·g−1,是未改性生物炭的7.9倍。PPy/MBC中带正电荷的氮(- nh·+-)、Mg2+和Cl−通过静电作用和离子交换去除地下水中的Cr(VI)。地下水中大部分阴离子和腐植酸对PPy/MBC对铬的去除效果不显著。总的来说,PPy/MBC复合材料的制备简单、绿色、环保。对地下水中Cr(VI)具有较高的吸附去除效率和吸附稳定性,对地下水中背景基质具有良好的抗干扰性。因此,它是一种很有前途的吸附剂,可以有效地处理地下水中的Cr(VI)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insights into the removal mechanism of Cr(VI) from groundwater by polypyrrole modified sludge biochar
Taking sludge biochar as the raw material and using iron and magnesium as metal sources respectively, two composite materials, polypyrrole magnesium modified biochar (PPy/MBC) and polypyrrole nano-zero-valent iron modified biochar (PPy/nZVI/BC), were prepared by in-situ oxidation polymerization of pyrrole monomers. The removal effects and mechanisms of these composite materials on hexavalent chromium (Cr(VI)) in groundwater were studied. The research results showed that the removal effect of PPy/MBC was superior to that of PPy/nZVI/BC. The highest removal rates of Cr(VI) and Cr(Total) were 170 and 120 mg·g−1 respectively. The adsorption kinetics followed the quasi-second-order kinetics and the adsorption isotherm conformed to the Langmuir adsorption isothermal model. The Elovich model indicated that the adsorption of Cr(VI) by PPy/MBC was a heterogeneous diffusion adsorption dominated by chemical adsorption. Thermodynamic analysis demonstrated that the adsorption of Cr(VI) by PPy/MBC was a spontaneous endothermic reaction. The specific surface area of the modified PPy/MBC reached 26.0824 m2·g−1, which was 7.9 times higher than that of the unmodified biochar. Positively charged nitrogen (-NH·+-), Mg2+ and Cl− in PPy/MBC removed Cr(VI) from groundwater through electrostatic interaction and ion exchange. Most anions and humic acids in groundwater had no significant effect on the removal of chromium by PPy/MBC. In general, the preparation of PPy/MBC composite material was simple, green and environmentally friendly. It had high adsorption removal efficiency and adsorption stability for Cr(VI) in groundwater, and good anti-interference against the background matrix in groundwater. Therefore, it was a promising adsorbent that could efficiently treat Cr(VI) in groundwater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


