N6-(2-羟乙基)-腺苷(HEA)通过调节IGF1信号传导,在骨肉瘤进展中表现出抗肿瘤活性
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
骨肉瘤是一种预后差的高度恶性骨肿瘤,由于耐药和副作用,治疗选择有限。目的探讨N6-(2-羟乙基)-腺苷(HEA)对骨肉瘤细胞的作用及其对IGF1信号通路的影响。方法用HEA处理saos2和MG63细胞株。评估细胞活力、凋亡、迁移、侵袭和EMT标志物。采用Western blot、qPCR和ELISA分析IGF1的表达。通过IGF1沉默和重组IGF1处理探讨HEA的机制。结果shea显著降低骨肉瘤细胞活力,诱导细胞凋亡,并呈剂量依赖性和时间依赖性。它还通过上调E-cadherin,下调N-cadherin和vimentin来抑制细胞迁移和侵袭,调节EMT标志物。HEA下调IGF1 mRNA和蛋白水平,减少IGF1分泌。此外,HEA抑制IGF1激活的PI3K-AKT信号通路。IGF1沉默模拟了HEA的作用,而重组IGF1预处理部分逆转了HEA对细胞活力、凋亡和EMT标志物的影响。结论shea通过靶向IGF1通路,抑制下游PI3K-AKT信号通路,对骨肉瘤细胞具有较强的体外和体内抗癌作用。这些结果表明HEA有望成为骨肉瘤的一种新型治疗剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
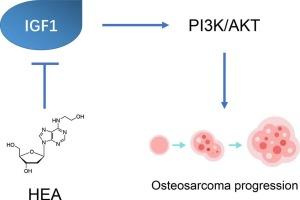
N6-(2-hydroxyethyl)-adenosine (HEA) exhibits antitumor activity for osteosarcoma progression by regulating IGF1 signaling
Background
Osteosarcoma is a highly malignant bone tumor with poor prognosis and limited treatment options due to resistance and side effects.
Objectives
This study investigates the effects of N6-(2-hydroxyethyl)-adenosine (HEA) on osteosarcoma cells and its impact on the IGF1 signaling pathway.
Methods
Saos2 and MG63 cell lines were treated with HEA. Cell viability, apoptosis, migration, invasion, and EMT markers were assessed. IGF1 expression was analyzed using Western blot, qPCR, and ELISA. IGF1 silencing and recombinant IGF1 treatments were used to explore HEA's mechanisms.
Results
HEA significantly decreased osteosarcoma cell viability and induced apoptosis in a dose- and time-dependent manner. It also inhibited cell migration and invasion, and modulated EMT markers by upregulating E-cadherin and downregulating N-cadherin and vimentin. HEA downregulated IGF1 at both the mRNA and protein levels, and reduced IGF1 secretion. Furthermore, HEA inhibited the PI3K-AKT signaling pathway, which is activated by IGF1. IGF1 silencing mimicked HEA's effects, whereas recombinant IGF1 pre-treatment partially reversed HEA's effects on cell viability, apoptosis, and EMT markers.
Conclusions
HEA exerts potent anti-cancer effects on osteosarcoma cells both in vitro and in vivo by targeting the IGF1 pathway and inhibiting downstream PI3K-AKT signaling. These results suggest that HEA holds promise as a novel therapeutic agent for osteosarcoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


