2- o系链烯基苯甲酸和二硒烯酸的电化学氧硒化合成1,4-苯并二苯二酮类化合物
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
建立了一种在室温条件下,以2- o系链烯基苯甲酸和二苯二烯为原料,在无氧化剂的条件下,电化学氧化硒化合成七元苯并二苯二酮的有效方法。实验证据支持这种转变是通过一种自由基机制实现的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
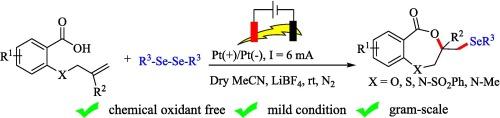
Synthesis of 1,4-benzodioxepinones via electrochemical oxyselenenylation of 2-O-tethered alkenyl benzoic acid and diselenides
An efficient methodology for the synthesis of seven-membered benzodioxepinones has been developed via electrochemical oxyselenenylation of 2-O-tethered alkenyl benzoic acid and diselenides under an external oxidant-free condition at room temperature. The experimental evidence supports this transformation through a radical mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


