一锅多步乌尔曼偶联反应高效合成高取代苯并硒唑衍生物
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在本研究中,以K2CO3附近的二卤苯为碱,以碘化铜为基料,在室温下,在MeCN溶剂中,采用一锅多步Ullman偶联反应,成功地合成了高取代苯并硒酸盐-硝基化合物的酰基异硒氰酸酯-硝基化合物加合物和二卤苯。采用简单易得的原料,温和的铜催化反应条件,容易借助溶剂提纯,最终合成了16个苯并硒化唑类化合物,是本方案的显著特点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
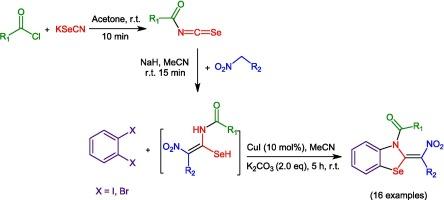
Efficient synthesis of highly substituted benzoselenazole derivatives through the one-pot, multi-step Ullmann coupling reaction
In this research, the simple and efficient method for the synthesis of highly substituted benzoselenazole derivatives using the one-pot, multi-step Ullman coupling reaction of acyl isoselenocyanate-nitro compounds adducts and dihalobenzene in the vicinity of K2CO3 as a base, copper iodide, at room temperature, and in MeCN solvent has been done successfully. The use of simple and available raw materials, mild copper catalytic reaction conditions, easy purification with the help of solvent, and finally the synthesis of 16 new compounds from the benzoselenazoles family are notable features of this protocol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


