钴对镍基/镁取代羟基磷灰石甲烷干重整催化剂活性的影响
IF 3.7
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
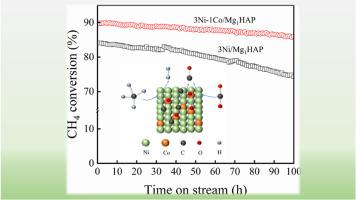
甲烷干重整(DRM)反应可以直接将甲烷(CH4)和二氧化碳(CO2)转化为合成气(H2+CO),是一种很有前途的实现碳中和的方法。本研究采用共沉淀法合成了一系列不同配比的3Ni-xCo/Mg1HAP合金催化剂,并对DRM反应的最佳Ni-Co配比进行了研究。一系列表征方法表明,加入Co后,Ni-Co合金的形成增加了金属间的相互作用。然而,过量的Co抑制了Ni进入Mg1HAP晶格,导致金属在支架表面堆积。此外,Co的引入改善了Ni金属的分散性,使催化剂具有更好的催化活性和稳定性。稳定性测试后的催化剂拉曼光谱显示,Co的加入降低了石墨碳的比例,这也是其稳定性提高的主要原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of cobalt on the activity of nickel-based/magnesium-substituted hydroxyapatite catalysts for dry reforming of methane
The dry reforming of methane (DRM) reaction can directly convert methane (CH4) and carbon dioxide (CO2) into syngas (H2+CO), which is a promising method for achieving carbon neutralization. In this study, a series of 3Ni-xCo/Mg1HAP alloy catalysts with different ratio were synthesized by the coprecipitation method, and the optimum Ni–Co ratio for the DRM reaction was studied. A series of characterization methods revealed that after Co was added, the formation of Ni–Co alloys increased the interactions between metals. However, an excess of Co inhibits the entry of Ni into the lattice of Mg1HAP, resulting in metal accumulation on the surface of the support. In addition, the introduction of Co improves the dispersion of Ni metal, which endows the catalyst with better catalytic activity and stability. Raman spectroscopy of the catalyst after the stability test showed that the addition of Co reduced the proportion of graphitic carbon, which was also the main reason for its improved stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Journal of Chemical Engineering
工程技术-工程:化工
CiteScore
6.60
自引率
5.30%
发文量
4309
审稿时长
31 days
期刊介绍:
The Chinese Journal of Chemical Engineering (Monthly, started in 1982) is the official journal of the Chemical Industry and Engineering Society of China and published by the Chemical Industry Press Co. Ltd. The aim of the journal is to develop the international exchange of scientific and technical information in the field of chemical engineering. It publishes original research papers that cover the major advancements and achievements in chemical engineering in China as well as some articles from overseas contributors.
The topics of journal include chemical engineering, chemical technology, biochemical engineering, energy and environmental engineering and other relevant fields. Papers are published on the basis of their relevance to theoretical research, practical application or potential uses in the industry as Research Papers, Communications, Reviews and Perspectives. Prominent domestic and overseas chemical experts and scholars have been invited to form an International Advisory Board and the Editorial Committee. It enjoys recognition among Chinese academia and industry as a reliable source of information of what is going on in chemical engineering research, both domestic and abroad.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


