MdO2的电子结构和化学键
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
采用完全相对论离散变分法(RDV)计算了MdO2的电子结构,建立了分子轨道(MO)格式,并在电子结合能(BE) 0 ~ 40 eV范围内建立了x射线光电子能谱(XPS)直方图。发现价电子XPS谱的复杂结构与外价电子轨道(OVMO, BE, 0 ~ 15ev)和内价电子轨道(IVMO, BE, ~ 15 ~ 40ev)的形成有关。由MO组成计算得出MdO2中Md的有效电荷为+0.50个电子。发现IVMO电子削弱了MdO2中OVMO形成的键约34%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
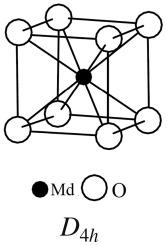
Electronic structure and chemical bond in MdO2
The calculation of the electronic structure of MdO2 was carried out using the fully relativistic method of discrete variation (RDV), a scheme of molecular orbitals (MO) was built and a histogram of the spectrum of X-ray photoelectron spectroscopy (XPS) was constructed in the range of electron binding energies (BE) 0–∼40 eV. It was discovered that the complex structure of the XPS spectrum of valence electrons is associated with the formation of the outer valence molecular orbitals (OVMO, BE from 0 to ∼15 eV) and of the inner valence molecular orbitals (IVMO, BE from ∼15 to ∼40 eV). The effective charge of Md in MdO2 calculated from the MO compositions was equal to +0.50 electrons. IVMO electrons were found to weaken the bond formed by OVMO in MdO2 by ∼34%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


