支链rac- c8n型碳糖如C7N碳糖的分子内aza-michael加成无金属催化剂β-胺化:生物进化、DFT研究和ADME性质
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
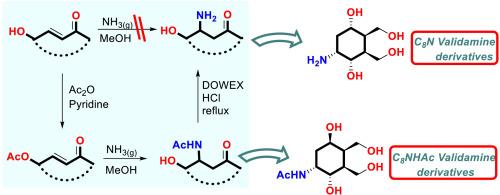
本研究以化合物4为起始原料,在甲醇中与α、β-不饱和酮和氨发生分子内aza-michael β-胺化反应,建立了一种新的立体定向制备C8N氨基环己醇的策略,如C7N、validamine类似物。该策略是通过Kornblum-DeLaMare重排制备C8N衍生物,如validamine C7N,该重排包括双键的立体控制胺化,酯化,羰基还原,苯并呋喃环开环,乙酸基氨解。讨论了靶分子的作用机理。在α-葡萄糖苷酶、β-葡萄糖苷酶和α-淀粉酶的作用下,研究了不同构型的假糖在端粒位置含有一个氨基。其中,化合物12对α-葡萄糖苷酶、化合物14对β-葡萄糖苷酶和化合物21对α-淀粉酶的活性较阿卡波糖最强。此外,对这些化合物进行了酶动力学研究以了解酶抑制机制和DFT研究以研究与酶活性位点的结合相互作用(12,14,21)。此外,使用QikProp模块检测药代动力学参数(ADME),以确定其作为候选药物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Metal catalyst-free β-amination of branched rac-C8N-type such as C7N carbasugars via intramolecular aza-michael addition: Biological evolution, DFT studies and ADME properties
In this study, a new stereospecific strategy for the preparation of C8N aminocyclohexenols such as C7N, validamine analogs were developed from starting compound 4 via intramolecular aza-michael β-amination reaction between α, β-unsaturated ketones and ammonia in methanol. The strategy was to produce C8N derivatives such as validamine C7N via Kornblum-DeLaMare rearrangement, which involves stereocontrolled amination of a double bond, esterification, carbonyl group reduction, benzofuran ring opening, ammonolysis of acetate groups. The mechanism of target molecules is discussed. Pseudosugars with different configurations containing an amino group at the anomeric position were tested against α-glucosidase, β-glucosidase, and α-amylase. Among these compounds, compound 12 against α-glucosidase, compound 14 against β-glucosidase, and compound 21 against α-amylase exhibited the best activity compared to acarbose. Moreover, enzyme kinetic studies to understand the enzyme inhibition mechanism and DFT studies to investigate binding interactions with enzyme active sites were performed on these compounds (12, 14, and 21). Additionally, the pharmacokinetic parameters (ADME) were examined using the QikProp module to determine their potential as drug candidates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


