Cdkn1a的缺失通过保持脂质稳态来单独或与酒精摄入一起预防MASLD
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景,由CDKN1A基因编码的P21的表达与脂肪变性肝病(SLD)的纤维化进展相关;然而,其潜在机制尚不清楚。在本研究中,我们研究了CDKN1A在SLD中的功能。方法在不同的SLD、纤维化和晚期慢性肝病(ACLD)患者队列中评估scdkn1a表达水平。Cdkn1a-/-和Cdkn1a+/+小鼠分别被喂食西方饮食(WD)、Lieber-DeCarli饮食(LdC)加多次乙醇(乙醇)狂欢,或双重饮食(代谢功能障碍相关的脂肪肝和酒精相关的肝脏)。分离原代肝细胞并进行功能测定。结果CDKN1A表达在脂肪性肝炎合并纤维化(与NAFLD活动度评分和纤维化分期评分呈正相关)、肝硬化合并ACLD患者中均显著升高。Cdkn1a+/+小鼠,喂食DuAL饮食表现出肝损伤和细胞死亡,活性氧(ROS)增加,衰老标志物(γH2AX, β-GAL, Cdkn1a/p53)导致脂肪变性和炎症。相比之下,Cdkn1a-/-突变小鼠显示出衰老相关标记物以及肝损伤、肝脂肪变性标记物的显著减少,脂肪酸氧化增加,游离脂肪酸摄取减少以及新生脂肪生成。机制上,在cdkn1a缺失的动物中观察到AMPK-SIRT3的激活。结论scdkn1a缺失通过AMPK-SIRT3轴促进脂肪酸氧化,阻止游离脂肪酸摄取和新生脂肪生成,从而预防临床前SLD。发现CDKN1A表达与SLD患者NAFLD活动评分和纤维化严重程度的增加直接相关。CDKN1A可能是治疗SLD患者代谢失调的潜在诊断靶点,无论是否饮酒。影响和意义:CDKN1A基因编码的p21的表达与脂肪变性肝病(SLD)的纤维化进展有关,但其分子机制尚不明确。有趣的是,在这项研究中,我们发现Cdkn1a缺失通过AMPK-SIRT3轴促进脂肪酸氧化,阻止游离脂肪酸摄取和新生脂肪生成,从而预防临床前SLD。翻译上,Cdkn1a的表达被发现与SLD患者NAFLD活动评分(NAS)和纤维化严重程度的增加直接相关,因此,Cdkn1a可能被用于治疗代谢诱导的SLD的潜在诊断靶点,无论是否饮酒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
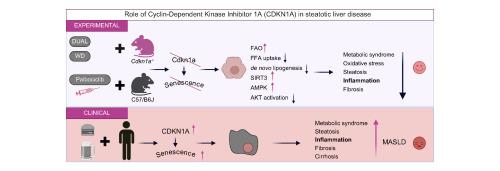
Loss of Cdkn1a protects against MASLD alone or with alcohol intake by preserving lipid homeostasis
Background & Aims
Expression of P21, encoded by the CDKN1A gene, has been associated with fibrosis progression in steatotic liver disease (SLD); however, the underlying mechanisms remain unknown. In the present study, we investigated the function of CDKN1A in SLD.
Methods
CDKN1A expression levels were evaluated in different patient cohorts with SLD, fibrosis, and advanced chronic liver disease (ACLD). Cdkn1a-/- and Cdkn1a+/+ mice were fed with either a Western diet (WD), a Lieber-DeCarli (LdC) diet plus multiple EtOH (ethanol) binges, or a DuAL diet (metabolic dysfunction-associated fatty liver disease and alcohol-related liver). Primary hepatocytes were isolated and functional assays performed.
Results
A significant increase in CDKN1A expression was observed in patients with steatohepatitis and fibrosis (with a positive correlation with both NAFLD Activity Score and fibrosis staging scores), cirrhosis and ACLD. Cdkn1a+/+ mice, fed a DuAL diet exhibited liver injury and cell death increased reactive oxygen species (ROS), and markers of senescence (γH2AX, β-GAL, Cdkn1a/p53) contributing to steatosis and inflammation. In contrast, Cdkn1a-/- mutant mice showed a significant decrease in senescence-associated markers as well as in markers of liver injury, hepatic steatosis and an increase in fatty acid oxidation and reduction in free fatty acid uptake as well as de novo lipogenesis. Mechanistically, activation of the AMPK-SIRT3 was observed in Cdkn1a-deleted animals.
Conclusions
Cdkn1a deletion protected against preclinical SLD by promoting fatty acid oxidation and preventing free fatty acid uptake and de novo lipogenesis via the AMPK-SIRT3 axis. CDKN1A expression was found to be directly correlated with increased severity of NAFLD Activity Score and fibrosis in patients with SLD. CDKN1A could be a potential theragnostic target for the treatment of metabolic dysregulation in patients with SLD, with and without alcohol consumption.
Impact and implications:
Expression of p21, encoded by the CDKN1A gene, has been associated with fibrosis progression in steatotic liver disease (SLD), but the molecular mechanisms remain elusive. Interestingly, in this study we found that Cdkn1a deletion protected against preclinical SLD by promoting fatty acid oxidation and preventing free fatty acid uptake and de novo lipogenesis, via the AMPK-SIRT3 axis. Translationally, Cdkn1a expression was found to be directly correlated with increased severity of NAFLD Activity Score (NAS) and fibrosis in SLD patients, and therefore, CDKN1A might be used potential theragnostic target for the treatment of metabolically induced SLD, with and without alcohol consumption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


