电负性差异调节双金属活性位点的协同作用,改善四环素的光降解
IF 4.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
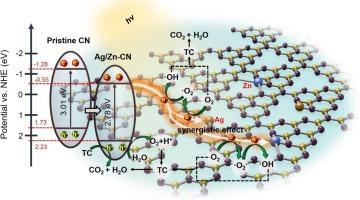
通过典型的金属原子锚定氮化碳(CN)载体来揭示双活性位点的协同效应是非常必要的,而可控的协同效应也是调节相邻配位环境的一种新途径。本文利用银、铁、锌原子的不同电负性,通过掺杂制备了一系列双活性位光催化剂。综合表征表明,包埋电负性差异显著的Ag/Zn杂原子可以调节双活性位点的协同效应,有利于调整电子结构,抑制光生电荷的重组,扩大可见光的吸收范围。然后,Ag和Zn双金属原子共掺杂CN (Ag/Zn-CN)光催化剂表现出比Ag/Fe-CN和Zn/Fe-CN更高的TC降解率(90 min时90.32%)和光电化学性能,在t = 0 ~ 50 min时一级动力学常数高达0.041 min−1。该工作为通过嵌入不同相邻原子来合理调节双金属活性位点光催化剂的配位环境提供了多种途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electronegativity difference regulates the synergistic effect of dual-metal active sites for improved photodegradation of tetracycline
It is extremely necessary to reveal the synergistic effect of dual-active sites via the typical metallic atoms anchored on carbon nitride (CN) carrier, and the controllable synergistic effect is also a novel approach to regulate the neighboring coordination environment. Herein, a series of dual-active site photocatalysts are papered by doping Ag, Fe or Zn atoms due to their different electronegativity. Comprehensive characterizations demonstrate that embedding of Ag/Zn heteroatoms with significant electronegativity difference can regulate the synergistic effect of dual-active sites, which is beneficial for tailoring electronic structures, suppressing the recombination of photogenerated charges, and expanding the absorption range of visible light. Then, the Ag and Zn dual-metal atoms co-doped CN (Ag/Zn-CN) photocatalyst exhibits higher TC degradation rate (90.32 % at 90 min) and photoelectrochemical performance than the Ag/Fe-CN and Zn/Fe-CN, the first-order kinetic constant up to 0.041 min−1 at t = 0 ∼ 50 min. This work provides a versatile avenue for the rational regulation of the coordination environment of dual-metal active site photocatalysts by embedding different neighboring atoms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


