Cu-ABNO催化剂用于醇类有氧双脱氢合成喹啉类和吡嗪类
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文合成了一种新的咪唑和酰胺功能化的钳状Cu(II)配合物(1)。以1和9-氮杂环[3.3.1]壬烷NH-Oxyl (ABNOH)为原料,探索了醇氧化及随后醇氧化引发的喹啉类和吡嗪类合成的催化方案。2-氨基醇等醇类也被有效氧化。由于2-芳氨基苯醇和仲醇的羰基是喹啉类化合物的合成物,因此我们探索了直接由醇合成它们的方法。该方案非常有效,仅在~ 5-10小时内完成反应。(a)伯2-芳基氨基苯甲酸醇与仲醇或其酮的组合,以及(b)伯2-芳基氨基苯甲酸醇与仲醇或其酮的组合,被发现对合成喹啉非常有效。该方案还成功地在10小时内由1,2-二醇和1,2-二氨基苯合成了各种吡嗪。机理研究表明,所生成的配合物起到了活性催化剂的作用:它激活O2,随后在9-氮杂环[3.3.1]壬烷n -氧(ABNO•)的配合下活化了配位醇酮的α-CH氢。然后,Cu(II)/Cu(I)还原生成羰基化合物,羰基化合物通过连续的C-C / C-N偶联反应在KOtBu和1存在下形成杂环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
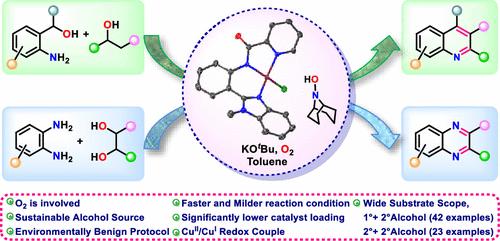
Cu–ABNO Catalyst for the Synthesis of Quinolines and Pyrazines via Aerobic Double Dehydrogenation of Alcohols
In this report, a new imidazole- and amide-functionalized pincer-like Cu(II) complex (1) was synthesized and characterized. By employing 1 and 9-azabicyclo[3.3.1]nonane NH-Oxyl (ABNOH), a catalytic protocol for alcohol oxidation and the subsequent alcohol oxidation-triggered synthesis of quinolines and pyrazines were explored. Alcohols such as 2-aminoaryl alcohols were also oxidized efficiently. As carbonyls from 2-arylaminobenzyl alcohols and secondary alcohols are synthons for quinolines, we explored their synthesis directly from alcohols. The protocol was quite efficient and completed the reaction in only ∼5–10 h. Combinations such as (a) primary 2-arylaminobenzyl alcohols with secondary alcohols or their ketones and (b) secondary 2-arylaminobenzyl alcohols with secondary alcohols or their ketones were found to be very effective for the synthesis of quinolines. The protocol was also successful for the synthesis of various pyrazines from 1,2-diols and 1,2-diaminobenzenes in 10 h. Mechanistic investigations showed that the generated complex acted as an active catalyst: it activated O2 and subsequently with the cooperation of 9-azabicyclo[3.3.1]nonane N-Oxyl (ABNO•) activated the α-CH hydrogen of coordinated alkoxide. Then, Cu(II)/Cu(I) reduction led to the formation of carbonyl compounds, which via successive C–C/C–N coupling reactions resulted in heterocycles in the presence of KOtBu and 1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


