揭示红细胞酵母中谷胱甘肽毒素 Grx4 的自噬降解过程
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
谷胱甘肽(Grxs)是一种热稳定的小蛋白,可作为依赖谷胱甘肽的多功能硫醇转移酶。最近的研究阐明了它们在调节细胞铁和铜平衡中的作用。为了进一步阐明它们的功能,我们采用了生物信息学和实验分析相结合的方法。在 S. pombe 中,已经发现了五个 Grxs。我们的研究利用多序列比对和保守结构域预测,发现 Grx4 及其同源物的 C 端具有一个谷胱甘肽结构域(GRX 结构域),N 端具有一个类似硫代毒素的结构域(TRX 结构域)。通过构建在组成型 cam1 启动子或其原生启动子调控下表达截短的 Grx4 的菌株,研究了 GRX 结构域和 TRX 结构域的功能作用。我们的研究结果表明,位于Grx4的TRX结构域内的两个Atg8相互作用基序(AIM)FLKI和FQEI足以在氮或铁饥饿条件下分别诱导自噬降解。这是在首次了解 Grxs 内 TRX 结构域功能方面取得的重大进展。此外,在铁饥饿条件下,Pcl1在Δatg5或Δatg8菌株中的表达水平发生了改变,这表明自噬对维持铁稳态至关重要。进一步研究发现,在 DTT 处理期间,细胞存活和内质网自噬(ER-phagy)需要 Grx4,这意味着 Grxs 与内质网(ER)之间存在潜在的相关性。此外,在ER应激过程中,Grx4的缺失会破坏核完整性,这凸显了进一步研究Grx4功能的多样性和重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
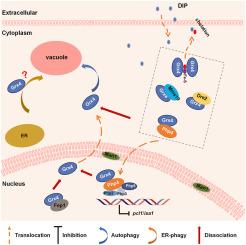
Schizosaccharomyces pombe Grx4 is subject to autophagic degradation under nitrogen- and iron- starvation and ER-stress
Glutaredoxins (Grxs) are small, heat-stable proteins that serve as multi-functional glutathione (GSH)-dependent thiol transferases. Recent studies have elucidated their role in regulating cellular iron and copper homeostases. In Schizosaccharomyces pombe, five Grxs (Grx1-5) have been identified. Among them, Grx4 and its homologs possess a C-terminal glutaredoxin domain (GRX) and an N-terminal thioredoxin-like domain (TRX). The functional roles of the GRX and TRX domains in Grx4 were investigated by constructing strains that express a truncated Grx4 under the regulation of either a constitutive cam1 promoter or its native promoter. Our findings indicated that two autophagy-related (Atg) protein 8 (Atg8)-interacting motifs (AIM), FLKI and FQEI, in the TRX domain of Grx4 are sufficient to induce autophagic degradation under nitrogen- and iron-starvation, respectively. Moreover, the expression level of a vacuolar ferrous iron transporter Pcl1 was altered in Δatg5 or Δatg8 strains under iron starvation,suggesting that autophagy is required for maintaining iron homeostasis in S. pombe. Further investigations revealed that Grx4 is required for cellular survival and endoplasmic reticulum (ER) autophagy (ER-phagy) during dithiothreitol (DTT) treatment, implying a potential correlation between Grxs and ER-stress. Additionally, loss of Grx4 disrupts nuclear integrity during ER stress, highlighting the versatility and importance of further investigations into the functions of Grx4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


