氟维罗松 A 的仿生合成
IF 1.7
4区 化学
引用次数: 0
摘要
在过去的一个世纪中,生物模拟合成大大提高了我们对天然产物形成过程中生物合成途径的认识。在这篇文章中,我们介绍了一种以 2,3-脱氢阿洛糖嘌呤为原料、以纳扎罗夫式环化为关键步骤的两步生物模拟合成氟维罗酮 A。根据我们的合成结果和计算分析,我们提出了氟维罗萨酮 A 的另一条生物合成路线,并确定丙酮醛可能是securinega 骨架中额外三个碳的来源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
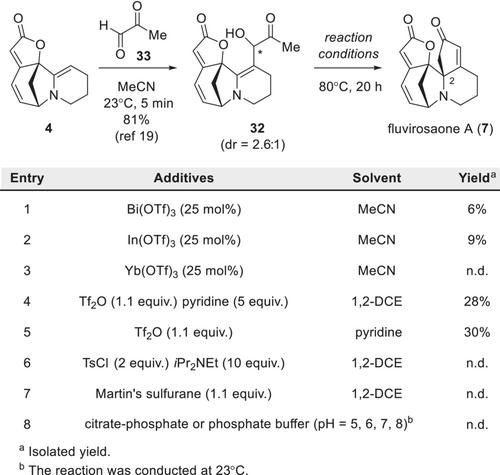
Biomimetic synthesis of fluvirosaone A
Over the past century, biomimetic synthesis has significantly enhanced our understanding of the biosynthetic pathways involved in the formation of natural products. In this article, we present a two-step biomimetic synthesis of fluvirosaone A from 2,3-dehydroallosecurinine, featuring a Nazarov-type cyclization as the key step. Based on our synthetic results and computational analysis, we propose an alternative biosynthetic route for fluvirosaone A, identifying pyruvaldehyde as the likely source of the extraneous three carbons incorporated into the securinega skeleton.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bulletin of the Korean Chemical Society
Chemistry-General Chemistry
自引率
23.50%
发文量
182
期刊介绍:
The Bulletin of the Korean Chemical Society is an official research journal of the Korean Chemical Society. It was founded in 1980 and reaches out to the chemical community worldwide. It is strictly peer-reviewed and welcomes Accounts, Communications, Articles, and Notes written in English. The scope of the journal covers all major areas of chemistry: analytical chemistry, electrochemistry, industrial chemistry, inorganic chemistry, life-science chemistry, macromolecular chemistry, organic synthesis, non-synthetic organic chemistry, physical chemistry, and materials chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


