利用铝土矿渣制备的铝基金属有机框架增强全氟辛酸的去除效果
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
将固体废弃物升级再利用为先进的吸附剂是废物价值化和废水处理领域的一种可持续方法。在本研究中,我们利用赤泥(RM)中的铝源(Al3+),开发了一种铝基金属有机框架(MOF)的单相相控合成方法,并证明了其在去除水性全氟辛酸(PFOA)方面的潜力。通过优化预处理过程,实现了从赤泥中选择性提取铝离子。随后,通过控制水热合成条件和使用特定的有机连接剂(对苯二甲酸和三聚甲酸),合成了三种不同的铝基 MOFs(即 MIL-53(Al)、MIL-96(Al)和 MIL-100(Al))。对于基于三美酸的 MOF,初始 Al3+:三美酸的比例和水热合成的持续时间对 MOF 第二构建单元的形成有明显的影响。通过调节这些因素,我们可以精确地控制分离出的 MIL-96(Al) 和 MIL-100(Al)。PFOA 的吸附结果表明,MIL-100(Al) 的吸附容量(Qmax:131.58 mg/g)比 MIL-96(Al)显著增加。这是因为它的表面积大(1189.15 m2/g),而且存在许多有利于与全氟辛烷磺酸的羧基相互作用的亲水位点。此外,计算调查显示,除了全氟辛烷磺酸和铝位点之间的直接路易斯酸碱相互作用外,主要机制还包括配位的 NO3- 和全氟辛烷磺酸阴离子之间的离子交换诱导形成的复合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
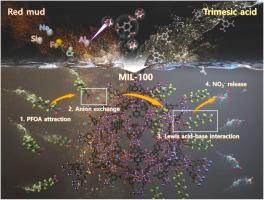
Enhanced removal of perfluorooctanoic acid by aluminum-based metal–organic frameworks prepared by bauxite residue
Upcycling solid waste into advanced adsorbents is a sustainable approach in the field of waste valorization and wastewater treatment. In this study, we developed a phase-controlled synthesis method for a single phase of an aluminum-based metal–organic framework (MOF) using an aluminum source (Al3+) in red mud (RM), and demonstrated its potential for aqueous perfluorooctanoic acid (PFOA) removal. By optimizing the pre-treatment process, the selective extraction of aluminum ion from RM was achieved. Subsequently, three distinct aluminum-based MOFs (i.e., MIL-53(Al), MIL-96(Al), and MIL-100(Al)) were synthesized by controlling the hydrothermal synthesis conditions and using specific organic linkers (terephthalic acid and trimesic acid). For MOFs based on trimesic acid, the initial Al3+: trimesic acid ratio and duration of hydrothermal synthesis exerted an observable influence on the formation of the second building unit of the MOF. By manipulating these factors, we could precisely control isolated MIL-96(Al) and MIL-100(Al). The PFOA adsorption results revealed a remarkable increase in the adsorption capacity (Qmax: 131.58 mg/g) on MIL-100(Al) compared with that on MIL-96(Al). This was due to its large surface area (1189.15 m2/g) and the presence of numerous hydrophilic sites favorable for interaction with the carboxylic group of PFOA. Furthermore, a computational investigation revealed that in addition to direct Lewis acid–base interaction between PFOA and aluminum sites, the major mechanism involved the formation of a complex induced by ion exchange between coordinated NO3- and PFOA anions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


