认知抗原参与诱导长期接受抗逆转录病毒治疗者的潜伏感染 CD4+ T 细胞表达 HIV-1
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
尽管采用了抗逆转录病毒疗法(ART),HIV-1 仍然存在于潜伏感染的 CD4+ T 细胞中,无法治愈。抗原促使受感染细胞增殖,从而阻止了潜伏库的衰减。然而,人们对抗原识别与 HIV-1 基因表达之间的关系知之甚少,因为大多数关于潜伏逆转的研究都使用了诱导非特异性全局 T 细胞活化的制剂。在这里,我们从长期接受抗逆转录病毒疗法的HIV-1感染者中分离出了对巨细胞病毒(CMV)或HIV-1 Gag抗原有反应的罕见CD4+T细胞,并评估了与呈现同源抗原的自体树突状细胞(DCs)共培养后的T细胞活化和HIV-1 RNA表达。体内递呈同源抗原可诱导广泛的T细胞活化(CD154+CD69+细胞中位数增加42倍),并显著增加HIV-1转录(中位数增加4倍),这主要是通过诱导病毒表达较高的稀有细胞实现的。因此,尽管病毒诱导性较低,但抗原识别可促进 HIV-1 的表达,从而有可能在抗逆转录病毒疗法中断后导致自发的储库活动和病毒反弹。本文章由计算机程序翻译,如有差异,请以英文原文为准。
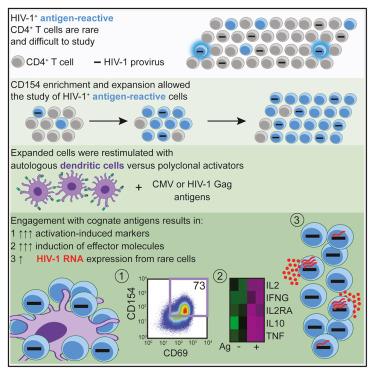
Cognate antigen engagement induces HIV-1 expression in latently infected CD4+ T cells from people on long-term antiretroviral therapy
Despite antiretroviral therapy (ART), HIV-1 persists in latently infected CD4+ T cells, preventing a cure. Antigens drive the proliferation of infected cells, precluding latent reservoir decay. However, the relationship between antigen recognition and HIV-1 gene expression is poorly understood because most studies of latency reversal use agents that induce non-specific global T cell activation. Here, we isolated rare CD4+ T cells responding to cytomegalovirus (CMV) or HIV-1 Gag antigens from people living with HIV-1 on long-term ART and assessed T cell activation and HIV-1 RNA expression upon coculture with autologous dendritic cells (DCs) presenting cognate antigens. Presentation of cognate antigens ex vivo induced broad T cell activation (median 42-fold increase in CD154+CD69+ cells) and significantly increased HIV-1 transcription (median 4-fold), mostly through the induction of rare cells with higher viral expression. Thus, despite low proviral inducibility, antigen recognition can promote HIV-1 expression, potentially contributing to spontaneous reservoir activity and viral rebound upon ART interruption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


