通过优化的脂质纳米颗粒配方输送核糖核蛋白,实现更安全高效的碱基编辑和基质编辑
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
为体内基因组编辑提供核糖核蛋白(RNPs)比使用编码 Cas9 及其相应引导 RNA 的病毒更安全。然而,瞬时的 RNP 活性通常不会带来最佳的编辑结果。在这里,我们展示了细胞穿透肽(与蛋白质共价融合或作为辅料)可以提高 RNP 的递送效率,并且可以优化封装 RNP 的脂质纳米颗粒(LNPs),以提高 RNP 的稳定性、递送效率和编辑效力。具体来说,在筛选了合适的可电离阳离子脂质并优化了合成脂质 DMG-PEG 2000 的浓度后,我们发现通过微流体混合将腺嘌呤碱基编辑物和质子编辑物 RNPs 封装在使用可电离脂质 SM102 的 LNPs 中,可使体内编辑效率提高 300 倍以上(与裸露 RNP 的递送相比),且不会出现可检测到的脱靶编辑。我们相信,针对 RNP 封装稳定性和递送效率进行优化的化学定义 LNP 配方将带来更安全的基因组编辑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
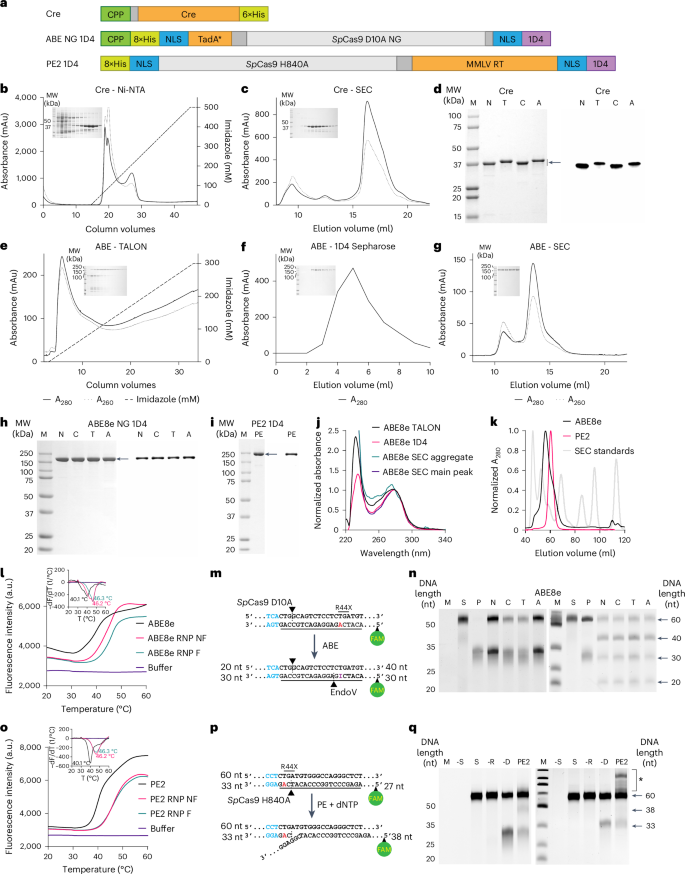

Safer and efficient base editing and prime editing via ribonucleoproteins delivered through optimized lipid-nanoparticle formulations
Delivering ribonucleoproteins (RNPs) for in vivo genome editing is safer than using viruses encoding for Cas9 and its respective guide RNA. However, transient RNP activity does not typically lead to optimal editing outcomes. Here we show that the efficiency of delivering RNPs can be enhanced by cell-penetrating peptides (covalently fused to the protein or as excipients) and that lipid nanoparticles (LNPs) encapsulating RNPs can be optimized for enhanced RNP stability, delivery efficiency and editing potency. Specifically, after screening for suitable ionizable cationic lipids and by optimizing the concentration of the synthetic lipid DMG-PEG 2000, we show that the encapsulation, via microfluidic mixing, of adenine base editor and prime editor RNPs within LNPs using the ionizable lipid SM102 can result in in vivo editing-efficiency enhancements larger than 300-fold (with respect to the delivery of the naked RNP) without detectable off-target edits. We believe that chemically defined LNP formulations optimized for RNP-encapsulation stability and delivery efficiency will lead to safer genome editing. The safety and efficacy of ribonucleoproteins for genome editing can be enhanced by formulations of lipid nanoparticles optimized for enhanced stability, delivery efficiency and editing potency of the protein complexes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


