循环中的肿瘤反应性 KIR+CD8+ T 细胞抑制黑色素瘤患者的抗肿瘤免疫力
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
有效的抗肿瘤免疫是由对肿瘤抗原具有特异性的细胞毒性 CD8+ T 细胞驱动的。然而,成功控制肿瘤排斥反应的因素尚不十分清楚。在这里,我们发现了一种 CD8+ T 细胞亚群,它们对肿瘤抗原具有特异性,可通过 KIR 表达进行识别,但却反常地损害了黑色素瘤患者的抗肿瘤免疫力。这些肿瘤抗原特异性 KIR+CD8+ 调节性 T 细胞靶向其他肿瘤抗原特异性 CD8+ T 细胞,可在肿瘤和血液中检测到,具有保守的转录程序,并与总生存率低有关。这些发现拓宽了我们对人类 CD8+ T 细胞转录和功能异质性的认识,并使 KIR+CD8+ 调节性 T 细胞成为人类癌症免疫逃避的细胞介质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
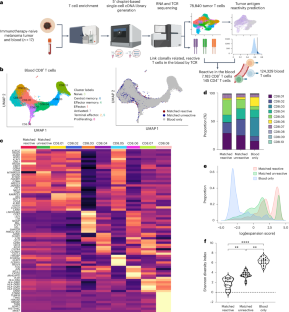

Circulating tumor-reactive KIR+CD8+ T cells suppress anti-tumor immunity in patients with melanoma
Effective anti-tumor immunity is driven by cytotoxic CD8+ T cells with specificity for tumor antigens. However, the factors that control successful tumor rejection are not well understood. Here we identify a subpopulation of CD8+ T cells that are tumor-antigen-specific and can be identified by KIR expression but paradoxically impair anti-tumor immunity in patients with melanoma. These tumor-antigen-specific KIR+CD8+ regulatory T cells target other tumor-antigen-specific CD8+ T cells, can be detected in both the tumor and the blood, have a conserved transcriptional program and are associated with a poor overall survival. These findings broaden our understanding of the transcriptional and functional heterogeneity of human CD8+ T cells and implicate KIR+CD8+ regulatory T cells as a cellular mediator of immune evasion in human cancer. Tumor-antigen-specific CD8+ T cells are generally thought to help fight against cancer, but here the authors identify a subpopulation of CD8+ T cells that are associated with a poor clinical outcome in melanoma. Although these cells can recognize tumor antigens, they suppress cancer immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


