piRNA 抵御内源性逆转录病毒的能力
摘要
逆转录病毒感染和转座元件的移动会造成 DNA 损伤,对细胞来说可能是灾难性的。如果细胞存活下来,逆转录产生的突变可能会带来选择性优势,但更常见的是,新整合子的影响是中性或有害的。如果逆转录发生在配子或早期胚胎中,它引入的遗传修饰可传递给后代,并可能固定在该物种的种系中。PIWI-interacting RNA(piRNA)是由 Argonaute 蛋白 PIWI 支系产生的 21-35 个核苷酸的单链 RNA,通过沉默转座子来维持动物种系的完整性。piRNA 和种系编码的 PIWI 蛋白抑制转座子的特定序列方式让人联想到 CRISPR,后者对入侵的病原体序列保持记忆。通过互补碱基配对,成熟的反义 piRNA 引导 Argonaute 蛋白的 PIWI 支系找到转座子 RNA 进行降解。此外,这些装载 piRNA 的 PIWI 蛋白被导入细胞核,通过启动组蛋白和 DNA 甲基化来调节转座子的共转录抑制。入侵生殖细胞的逆转录病毒是如何首先被 piRNA 机制识别为外来的,以及靶向入侵遗传因子序列的内源性 piRNA 簇是如何获得的,目前尚不清楚。目前,考拉(Phascolarctos cinereus)正在经历一场由于 KoRV-A gammaretrovirus 的水平和垂直传播而导致的流行病。这为研究外源逆转录病毒如何固定在宿主的基因组中,以及受感染动物的生殖细胞如何产生针对这种逆转录病毒的 piRNA 提供了前所未有的机会。初步实验表明,考拉睾丸中来自 KoRV-A 前病毒的未剪接转录本(而非剪接的 KoRV-A 转录本)可直接加工成有义链 piRNA。未拼接的有义链转录本的裂解被认为是最初的先天性防御,直到产生反义 piRNA 并建立起适应性的 KoRV-A 特异基因组免疫反应。进一步的研究有望确定 piRNA 机制如何识别新的外来基因入侵者,如何区分有剪接和无剪接转录本,以及如何通过有义和无义 piRNA 以及病毒启动子处组蛋白和 DNA 的甲基化建立成熟的基因组免疫反应。
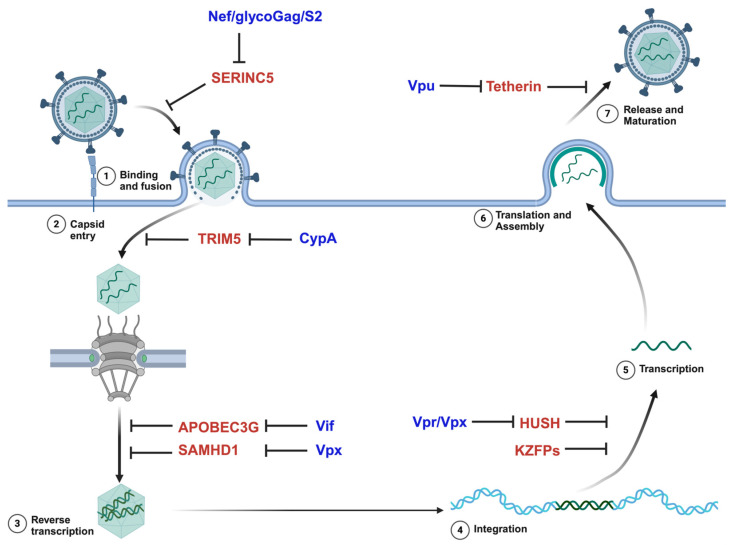
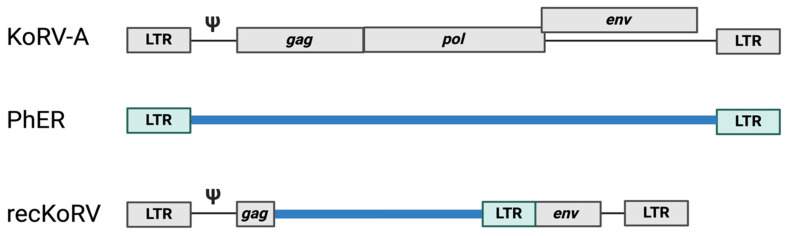
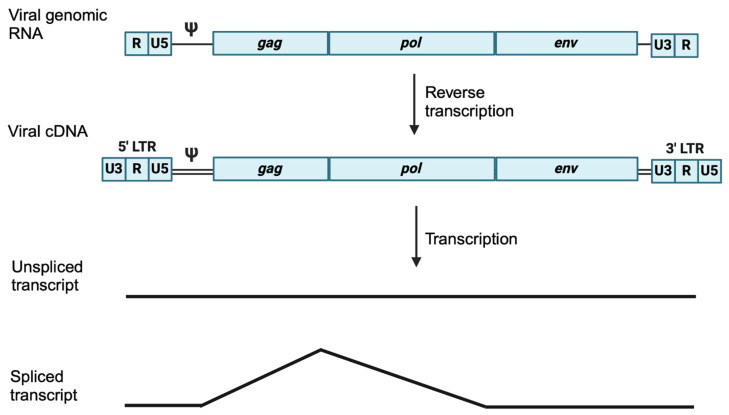
Infection by retroviruses and the mobilization of transposable elements cause DNA damage that can be catastrophic for a cell. If the cell survives, the mutations generated by retrotransposition may confer a selective advantage, although, more commonly, the effect of new integrants is neutral or detrimental. If retrotransposition occurs in gametes or in the early embryo, it introduces genetic modifications that can be transmitted to the progeny and may become fixed in the germline of that species. PIWI-interacting RNAs (piRNAs) are single-stranded, 21-35 nucleotide RNAs generated by the PIWI clade of Argonaute proteins that maintain the integrity of the animal germline by silencing transposons. The sequence specific manner by which piRNAs and germline-encoded PIWI proteins repress transposons is reminiscent of CRISPR, which retains memory for invading pathogen sequences. piRNAs are processed preferentially from the unspliced transcripts of piRNA clusters. Via complementary base pairing, mature antisense piRNAs guide the PIWI clade of Argonaute proteins to transposon RNAs for degradation. Moreover, these piRNA-loaded PIWI proteins are imported into the nucleus to modulate the co-transcriptional repression of transposons by initiating histone and DNA methylation. How retroviruses that invade germ cells are first recognized as foreign by the piRNA machinery, as well as how endogenous piRNA clusters targeting the sequences of invasive genetic elements are acquired, is not known. Currently, koalas (Phascolarctos cinereus) are going through an epidemic due to the horizontal and vertical transmission of the KoRV-A gammaretrovirus. This provides an unprecedented opportunity to study how an exogenous retrovirus becomes fixed in the genome of its host, and how piRNAs targeting this retrovirus are generated in germ cells of the infected animal. Initial experiments have shown that the unspliced transcript from KoRV-A proviruses in koala testes, but not the spliced KoRV-A transcript, is directly processed into sense-strand piRNAs. The cleavage of unspliced sense-strand transcripts is thought to serve as an initial innate defense until antisense piRNAs are generated and an adaptive KoRV-A-specific genome immune response is established. Further research is expected to determine how the piRNA machinery recognizes a new foreign genetic invader, how it distinguishes between spliced and unspliced transcripts, and how a mature genome immune response is established, with both sense and antisense piRNAs and the methylation of histones and DNA at the provirus promoter.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


