评估 2021-2022 年全球脊髓灰质炎实验室网络外部分子能力测试的有效性。
摘要
在全球脊灰病毒实验室网络(GPLN)中,参与并成功完成年度能力验证(PT)小组的工作是世卫组织认证流程的一部分,已有数十年的历史。能力验证小组是一项分子外部质量评估(mEQA),用于评估实验室的准备情况、技术熟练程度、数据解读的准确性和结果报告。实验室使用疾病预防控制中心(CDC)提供的实时 RT-PCR 检测试剂盒进行筛选检测,并根据 ITD 算法报告结果,以鉴定脊髓灰质炎病毒并对其进行分型。mEQA 面板由 10 个盲法非感染性冻干 RNA 转录本组成,包括与程序相关的病毒和实时 PCR 检测中包含的靶标。样本鉴定包括野生型、疫苗衍生型(VDPV)、Sabin 样脊髓灰质炎病毒、肠道病毒、阴性以及无效和不确定类别。根据实验室正确检测和确定每个样本的血清型/基因型特征的能力来评估各个实验室的绩效。评分标准按照 GPLN 指南评估实验室的准备情况。获得 90 分或以上 mEQA 分数的实验室通过评估,低于 90 分的实验室不合格,需要采取补救措施并重新评估。2021 年和 2022 年,分别有 123 家和 129 家 GPLN 实验室受邀申请年度 PT 小组评估,并分别有 118 家和 127 家实验室提交了评估结果。总体结果良好,2021 年和 2022 年分别有 86% 和 91.5% 的实验室首次尝试就通过了能力考评小组的考评。大多数实验室都获得了 100 分的最高分,只有不到四分之一的实验室得分在 90 分至 95 分之间。只有不到 10%的提交实验室未能通过测试,因此需要进行深入的故障排除,以找出根本原因并采取补救措施。这些实验室中的大多数都获得了第二份能力测试面板,用于重复测试,几乎所有实验室都通过了重复能力测试面板。2021 年和 2022 年的年度 mEQA 试验结果显示,尽管发生了 COVID-19 大流行,但 GPLN 的绩效仍然很高,大多数实验室都获得了最高分。对于这些实验室而言,2021-2022 年期间实施的实时 PCR 检测更新完全按照程序和算法进行。即使是最初不及格的实验室,在经过补救后也取得了及格分数。
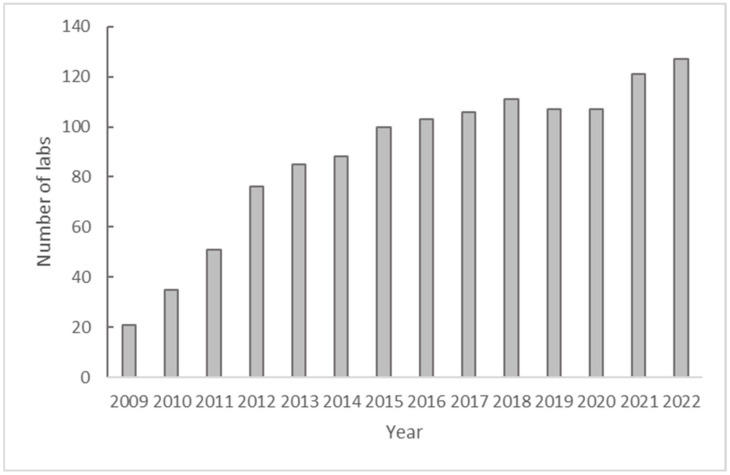
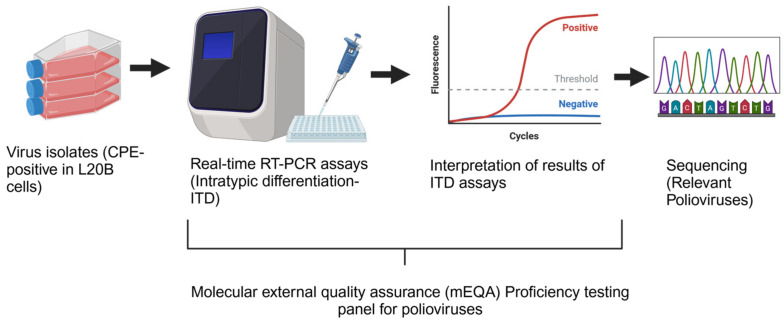
In the Global Poliovirus Laboratory Network (GPLN), participation and successful completion in annual proficiency test (PT) panels has been a part of the WHO accreditation process for decades. The PT panel is a molecular external quality assessment (mEQA) that evaluates laboratory preparedness, technical proficiency, the accuracy of data interpretation, and result reporting. Using the Intratypic Differentiation (ITD) real-time RT-PCR kits from CDC, laboratories run screening assays and report results in accordance with the ITD algorithm to identify and type polioviruses. The mEQA panels consisted of 10 blinded, non-infectious lyophilized RNA transcripts, including programmatically relevant viruses and targets contained in the real-time PCR assays. Sample identities included wildtype, vaccine-derived (VDPV), Sabin-like polioviruses, enterovirus, and negatives, as well as categories of invalid and indeterminate. The performance of individual laboratories was assessed based on the laboratory's ability to correctly detect and characterize the serotype/genotype identities of each sample. The scoring scheme assessed the laboratory readiness following GPLN guidelines. Laboratories receiving mEQA scores of 90 or higher passed the assessment, scores of less than 90 failed and required remedial actions and re-evaluation. In 2021 and 2022, 123 and 129 GPLN laboratories were invited to request the annual PT panel, and 118 and 127 laboratories submitted results, respectively. The overall results were good, with 86% and 91.5% of laboratories passing the PT panel on their first attempt in 2021 and 2022, respectively. Most labs scored the highest score of 100, and less than one quarter scored between 90 and 95. Less than 10% of submitting laboratories failed the PT, resulting in in-depth troubleshooting to identify root causes and remediations. Most of these laboratories were issued a second PT panel for repeat testing, and almost all laboratories passed the repeat PT panel. The results of the 2021 and 2022 annual mEQA PTs showed that, despite the COVID-19 pandemic, the performance remained high in the GPLN, with most labs achieving the highest score. For these labs, the real-time PCR assay updates that were implemented during 2021-2022 were carried out with full adherence to procedures and algorithms. Even initially failing labs achieved passing scores after remediation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


