高频经颅磁刺激对疼痛预期和感知的镇痛效果
IF 5.1
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
先前的研究表明,疼痛的感知在很大程度上受预期的影响,M1 和 DLPFC 参与了这一过程。我们假设,以这些区域为靶点的高频经颅磁刺激可以改变疼痛预期,从而降低痛觉。在一项双盲、假对照研究中,健康参与者接受了针对 M1、DLPFC 的 10 Hz 经颅磁刺激或假治疗。分别在刺激前、刺激后和刺激后 60 分钟进行评估,包括激光诱发电位、疼痛评分和预期脑电图。M1-经颅磁刺激立即降低了激光诱发电位P2振幅,增加了感觉运动高频α振荡功率,并加快了疼痛预感时中额区α频率峰值。与此相反,DLPFC-经颅磁刺激在刺激后60分钟降低了N2-P2复合体和疼痛评级,这种效应与疼痛预期期间微状态C持续时间延长有关--微状态与默认模式网络活动有关。因此,M1经颅磁刺激可立即调节预期α振荡和激光诱发电位,而DLPFC经颅磁刺激则部分通过调节默认模式网络活动诱导延迟镇痛效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
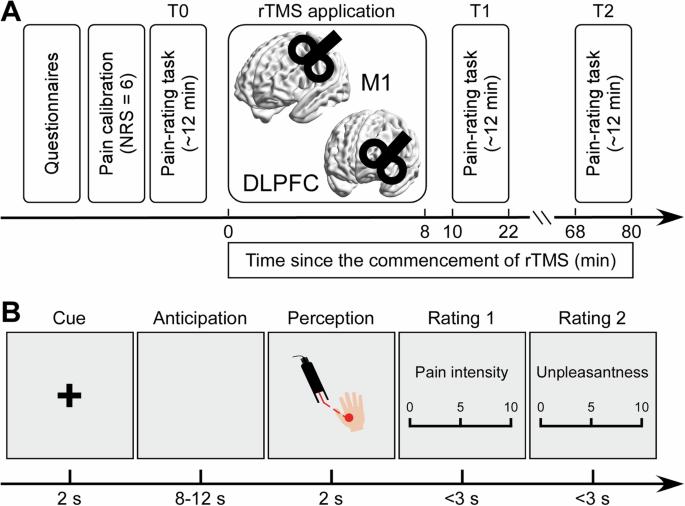
Analgesic effects of high-frequency rTMS on pain anticipation and perception
Previous studies suggest that pain perception is greatly shaped by anticipation, with M1 and DLPFC involved in this process. We hypothesized that high-frequency rTMS targeting these regions could alter pain anticipation and thereby reduce pain perception. In a double-blind, sham-controlled study, healthy participants received 10 Hz rTMS to M1, DLPFC, or a sham treatment. Assessments were conducted before, immediately after, and 60 min after stimulation, including laser-evoked potentials, pain ratings, and anticipatory EEG. M1-rTMS immediately reduced laser-evoked P2 amplitude, increased sensorimotor high-frequency α-oscillation power, and accelerated peak alpha frequency in the midfrontal region during pain anticipation. In contrast, DLPFC-rTMS reduced the N2-P2 complex and pain ratings 60 min post-stimulation, an effect associated with prolonged microstate C duration during pain anticipation—a microstate linked to default mode network activity. Thus, M1-rTMS immediately modulates anticipatory α-oscillations and laser-evoked potentials, while DLPFC-rTMS induces delayed analgesic effects partially by modulating default mode network activity. High-frequency rTMS to M1 and DLPFC modulates pain anticipation and perception. M1-rTMS immediately reduced pain via α-oscillation modulation, while DLPFC-rTMS produced delayed analgesia through default mode network modulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


