卡尔曼诺的聚合全合成。
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
Kalmanol (1) 是第一个分离出的卡尔曼型灰岩类化合物,具有高度氧化的 5/8/5/5 四环碳骨架和 9 个连续的立体中心。我们采用模块化合成策略,从已知化合物出发,经过 16 个步骤(从市售起始原料出发,经过 20 个步骤)完成了 1 的高效不对称全合成。通过格氏反应和随后的两个对映体富集片段的闭环偏合成反应,以聚合方式制备出四环中间体。多羟基是通过后期的立体和区域选择性氧化反应引入的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
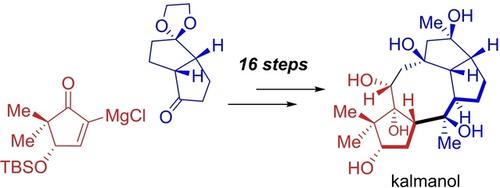
Convergent Total Synthesis of Kalmanol
Kalmanol (1) is the first isolated kalmane-type grayanoid featuring a highly oxidized 5/8/5/5 tetracyclic carbon skeleton and 9 contiguous stereocenters. We have accomplished the efficient and asymmetric total synthesis of 1 in 16 steps from known compounds (20 steps from commercially available starting materials) by a modular synthetic strategy. A tetracyclic intermediate was prepared in a convergent manner through a Grignard reaction and a subsequent ring-closing metathesis reaction of two enantiomerically enriched fragments. The polyhydroxy groups were introduced by late-stage stereo- and regioselective oxidations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


