铱催化的串联烯化/aza-迈克尔反应:快速获得 N-N 官能化肼。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种在温和条件下由 Ir 催化的受保护苯甲酰肼衍生物与烯烃的串联烯化/氮杂迈克尔反应。该方法可成功用于构建各种带有 α、β-不饱和侧链的 N-N 功能化酰肼衍生物,收率良好甚至极佳。特别是,脱氨基保护产物可用作构建 N-N 轴手性化合物的潜在前体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
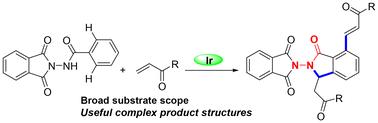
Iridium-catalyzed tandem olefination/aza-Michael reaction: rapid access to N–N functionalized hydrazides†
An Ir-catalyzed tandem olefination/aza-Michael reaction of protected benzoylhydrazine derivatives with olefins under mild conditions has been developed. This method can be successfully applied to the construction of various structurally N–N-functionalized hydrazide derivatives bearing the α,β-unsaturated side chain in good to excellent yields. In particular, the deaminoprotected products can be used as potential precursors for the construction of N–N axially chiral compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


