用于储能的纳米级 Li2FeS2 的另一种合成途径。
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
富锂硫化铁(Li2FeS2)通过阳离子和阴离子位点表现出可逆的电荷存储能力,可存储近 400 mA h g-1,但其合成仅限于固态方法,会产生较大的原生颗粒。我们介绍了另一种基于溶液、以氧化还原为介导的方法来使黄铁矿 FeS2 锂化,最终形成纳米级的 Li2FeS2。本文章由计算机程序翻译,如有差异,请以英文原文为准。

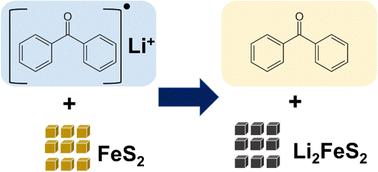
An alternate synthetic pathway to nanoscopic Li2FeS2 for energy storage†
Lithium-rich iron sulphide, Li2FeS2, exhibits reversible charge-storage via both cationic and anionic sites, storing nearly 400 mA h g−1, but its synthesis is limited to solid-state methods that result in large primary particles. We describe an alternate solution-based, redox-mediated method to lithiate pyrite FeS2, ultimately forming nanoscale Li2FeS2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


