一锅室温构建嵌入 g-C3N4 的氧溴化铋(BiOBr):在阳光直射下降解染料和还原铬 (VI) 的异质结光催化剂
IF 4.2
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
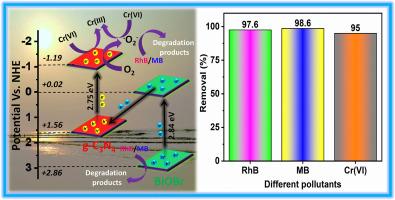
近年来,利用半导体光催化剂处理受污染的水(包括无机和有机污染物)的做法越来越受欢迎。然而,光催化剂仍然受到表面活性位点有限、电荷载流子过度重组和可见光消耗差等问题的制约。本文通过一锅室温自结合技术有效地制备了嵌入 g-C3N4 异质结的纳米氧化铋(BiOBr)光催化剂。随后,研究人员采用多种策略来研究异质结光催化剂的构建如何影响其消除不同污染物的能力。在阳光的直接照射下,20%的BiOBr/g-C3N4(BCN-20)能在80分钟内降解97.6%的RhB和98.6%的MB,并在90分钟后降低95%的六(六)铬。此外,BCN-20 异质结的降解效率和还原能力均优于 g-C3N4 和 BiOBr。 此外,BCN-20 光催化剂具有更高的稳定性。活性的提高可能是由于更紧密的界面相互作用、更高的表面积以及光诱导电子和空穴的强分离。根据实验结果,提出了一个 Z 型框架来阐明电荷载流子转移过程。根据自由基捕获测试,光催化分解 RhB 和 MB 染料的主要活性物质是空穴(h+)和超氧自由基(-O2-)。此外,在光催化还原六价铬时,主要的活性物质是-O2-和电子(e-)。本文介绍了一种用于环境及相关问题的光催化剂的简易制造工艺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
One-pot room temperature construction of bismuth oxybromide (BiOBr) embedded g-C3N4 matters: A heterojunction photocatalyst for dye degradation and chromium (VI) reduction in presence of direct sunlight
The use of semiconductor photocatalysts for the treatment of contaminated water, including inorganic and organic contaminants, has gained popularity in recent years. However, the photocatalysts are still constrained by issues such as limited surface active sites, excessive charge carrier recombination, and poor visible light consumption. Herein, bismuth oxybromide (BiOBr) nanoplate embedded g-C3N4 heterojunction photocatalyst was effectively fabricated via a one-pot room temperature self-association technique. Then, numerous strategies were used to figure out how the building of the heterojunction photocatalysts affected their ability to eliminate different pollutants. Under direct sunshine exposure, 20 % BiOBr/g-C3N4 (BCN-20) was able to degrade 97.6 % of RhB and 98.6 % of MB in 80 min and also reduced 95 % Cr(VI) after 90 min. Moreover, the degradation efficiency and reduction capability of BCN-20 heterojunction is superior compared to both g-C3N4 and BiOBr. Additionally, the BCN-20 photocatalyst has much greater stability. The increased activity may be due to the tighter interface interaction, the higher surface area, and the very strong separation of photo-induced electrons and holes. Based on experimental findings, a Z-scheme framework has been suggested to elucidate the charge carrier transfer process. The primary active substances for the photocatalytic breakdown of RhB and MB dye, according to the radical trapping tests, are holes (h+) and superoxide radicals (•O2−). Also, for photocatalytic reduction of Cr (VI), the primary active species are •O2− and electron (e−). This paper describes an easy process to fabricate photocatalysts for usage in environmental and associated issues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Optical Materials
工程技术-材料科学:综合
CiteScore
6.60
自引率
12.80%
发文量
1265
审稿时长
38 days
期刊介绍:
Optical Materials has an open access mirror journal Optical Materials: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The purpose of Optical Materials is to provide a means of communication and technology transfer between researchers who are interested in materials for potential device applications. The journal publishes original papers and review articles on the design, synthesis, characterisation and applications of optical materials.
OPTICAL MATERIALS focuses on:
• Optical Properties of Material Systems;
• The Materials Aspects of Optical Phenomena;
• The Materials Aspects of Devices and Applications.
Authors can submit separate research elements describing their data to Data in Brief and methods to Methods X.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


