一锅合成具有不同噻吩环的供体-受体氮化碳,加速光催化氢气进化
IF 3.8
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
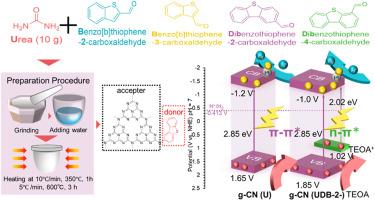
通过尿素与四种不同的噻吩基前驱体在大气条件下煅烧,合成了具有供体-受体结构的氮化石墨碳(g-CN),并在末端位点交联了噻吩基前驱体。其中,含有二苯并噻吩-2-羧基甲醛(DB-2)1 mg 的 UDB-2-1 显示出 650 μmol/g-h 的氢演化活性,比原始氮化石墨碳(U)高出约六倍。在磷酸氢钾(KPH)的作用下,在 400 纳米、420 纳米和 450 纳米波长处测得的表观量子产率(AQY)分别为 4.01%、4.89% 和 3.72%。性能的提高归因于噻吩环引入的 n-π∗ 转变增加了对可见光的吸收,以及供体-受体(DA)结构增强了电荷分离,其中 DB-2 充当了电子供体。制备的光催化剂通过 XRD、XPS、傅立叶变换红外光谱、扫描电镜、TEM、BET、ESR、EIS、Mott-Schottky、DRS、PL 和 TRPL 测量进行了表征。这项研究为提高氮化碳的性能提供了一种简单而有效的策略,并强调了官能团定位和结构异构体在太阳能应用分子设计中的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
One pot synthesis of donor-acceptor carbon nitride with distinct thiophene rings accelerate photocatalytic hydrogen evolution
Graphitic carbon nitride (g-CN) with a donor-acceptor structure was synthesized by urea and cross-linking thiophene precursors at the terminal sites, achieved through calcination of urea with four different thiophene-based precursors under atmospheric conditions. Among these, UDB-2-1, which incorporates dibenzothiophene-2-carboxyaldehyde (DB-2) 1 mg, demonstrated a hydrogen evolution activity of 650 μmol/g·h, approximately six times higher than pristine graphitic carbon nitride (U). The apparent quantum yield (AQY) was measured to be 4.01 %, 4.89 %, and 3.72 % at 400 nm, 420 nm, and 450 nm, respectively, in the presence of potassium hydrogen phosphate (KPH). This enhanced performance is attributed to increased visible light absorption from the n-π∗ transition introduced by the thiophene ring and enhanced charge separation due to the donor-acceptor (DA) structure, with DB-2 acting as an electron donor. Prepared photocatalysts were characterized by XRD, XPS, FTIR, SEM, TEM, BET, ESR, EIS, Mott-Schottky, DRS, PL, and TRPL measurement. This study provides a simple and effective strategy to improve carbon nitride performance and underscores the importance of functional group positioning and structural isomers in molecular design for solar-energy applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Optical Materials
工程技术-材料科学:综合
CiteScore
6.60
自引率
12.80%
发文量
1265
审稿时长
38 days
期刊介绍:
Optical Materials has an open access mirror journal Optical Materials: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The purpose of Optical Materials is to provide a means of communication and technology transfer between researchers who are interested in materials for potential device applications. The journal publishes original papers and review articles on the design, synthesis, characterisation and applications of optical materials.
OPTICAL MATERIALS focuses on:
• Optical Properties of Material Systems;
• The Materials Aspects of Optical Phenomena;
• The Materials Aspects of Devices and Applications.
Authors can submit separate research elements describing their data to Data in Brief and methods to Methods X.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


