氨基和酰胺基对荧光、粘度敏感、热稳定性和 NLO 发光的噻吩融合苊衍生物的影响
IF 3.8
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
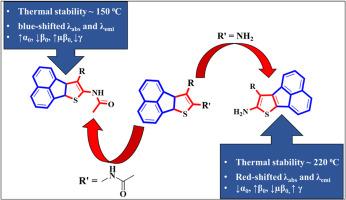
为了研究氨基和酰胺基团对几何、热、光谱、线性和非线性光学特性的影响,我们合成了基于 D-π-A 框架的噻吩融合苊衍生物。这些分子在甲苯、CHCl3、EA、THF、MeOH 和 DMSO 中的吸收⁓ 300-520 nm,发射⁓ 450-620 nm。此外,这些分子在甲苯-石蜡和 EtOH-PEG-400 体系中的发射强度增加了 ⁓ 1.20-5.67 倍。在 B3LYP/6-311++G(d,p) 下进行的 DFT 研究表明,这些分子是具有平面几何形状的富电子系统。TD-DFT 结果显示与实验吸收最大值相当吻合。噻吩融合苊衍生物具有热稳定性(⁓ 220-250 °C),与对硝基苯胺相比,其静态极化率和超极化率相对较高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of amino and amido group on fluorescent, viscosity sensitive, thermally stable, NLOphoric thiophene fused acenaphthene derivatives
Thiophene fused acenaphthene derivatives based on the D-π-A framework were synthesized to study the effect of amino and amido groups on geometrical, thermal, spectral, linear, and non-linear optical properties. These molecules exhibit absorption ⁓ 300–520 nm and emission ⁓ 450–620 nm range in toluene, CHCl3, EA, THF, MeOH, and DMSO. Moreover, these molecules exhibit ⁓ 1.20–5.67 fold increased emission intensity in the toluene-paraffin and EtOH-PEG-400 system. DFT study at B3LYP/6–311++G(d,p) revealed that these molecules are electron-rich systems with a planar geometry. TD-DFT results show a reasonable agreement with the experimental absorption maxima. Thiophene fused acenaphthene derivatives are thermal stable (⁓ 220–250 °C) and display relatively higher static polarizability and hyperpolarizability in comparison to p-nitroaniline.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Optical Materials
工程技术-材料科学:综合
CiteScore
6.60
自引率
12.80%
发文量
1265
审稿时长
38 days
期刊介绍:
Optical Materials has an open access mirror journal Optical Materials: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The purpose of Optical Materials is to provide a means of communication and technology transfer between researchers who are interested in materials for potential device applications. The journal publishes original papers and review articles on the design, synthesis, characterisation and applications of optical materials.
OPTICAL MATERIALS focuses on:
• Optical Properties of Material Systems;
• The Materials Aspects of Optical Phenomena;
• The Materials Aspects of Devices and Applications.
Authors can submit separate research elements describing their data to Data in Brief and methods to Methods X.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


