用于锂离子电池的具有先进性能的层状封闭离子液体电解质
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
离子液体有望取代传统的有机溶剂,为锂离子电池开发安全的电解质。然而,基于离子液体的电解质仍然存在性能低下和潜在泄漏的问题。在此,研究人员将非离子表面活性剂Brij58引入到由1-丁基-1-甲基吡咯烷鎓双酯酰亚胺(BMPTFSI)和LiTFSI盐组成的离子液体电解质(BMPTFSI/LiTFSI)中,构建了固态溶胀性液晶(LLC)。Brij58 在离子液体电解质中的自组装构建了薄片状纳米结构,它限制了离子液体电解质形成分层导电通道。固态 LLC 电解质中的纳米级层状导电通道具有类似液体的离子导电性,显著改善了锂离子的传输。虽然LLC电解质只消耗了50 wt %的BMPTFSI/LiTFSI,但与含有原始BMPTFSI/LiTFSI的锂离子电池相比,基于LLC电解质的锂离子电池的放电容量和速率性能有了很大提高。此外,片状 LLC 电解质还保留了阻燃特性。这项工作为通过构建片状各向同性有限责任公司纳米结构来优化IL电解质的导电性能铺平了新的道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
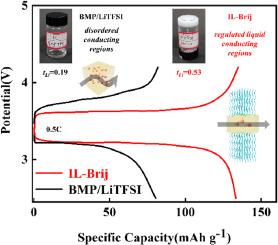
Lamellar confined ionic liquid electrolytes with advanced performance for Li-ion batteries
Ionic liquids are promising to rival traditional organic solvent to develop safe electrolytes for Li-ion batteries. However, the ionic liquid-based electrolytes still suffered from issues of inferior performance and potential leakage. Here, non-ionic surfactant Brij58 was introduced into ionic liquid electrolytes (BMPTFSI/LiTFSI) consisting of 1-butyl-1-methylpyrrolidinium bistriflimide (BMPTFSI) and LiTFSI salt to construct solid-state lyotropic liquid crystals (LLCs). Self-assembly of Brij58 in ionic liquid electrolytes constructed lamellar nanostructures, which confined the ionic liquid electrolytes to form layered conducting channels. The nanoscale layered conducting channels in solid-state LLC electrolytes endowed liquid-like ion conductivity and significantly improved Li-ion transfer. Although the LLC electrolytes only consumed 50 wt % BMPTFSI/LiTFSI, the discharge capacities and rate performance of the Li-ion batteries based on LLC electrolytes was much improved comparing to that containing pristine BMPTFSI/LiTFSI. Also, the flame-retardant feature retained within the lamellar LLC electrolytes. The work paved new way for optimizing the conducting performance of IL electrolytes by constructing lamellar lyotropic LLC nanostructures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


