拟合加速度量热数据的化学反应神经网络
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
随着对锂离子电池需求的快速增长,有必要以安全的方式设计这些电池,以缓解热失控现象。电池中的热失控会导致无法控制的温度上升,并可能引发电池火灾,这是一个重大的安全问题。通常情况下,在对热失控的化学动力学进行建模时,需要使用量热数据(如加速速率量热法 (ARC))来确定温度驱动的分解动力学。将阿伦尼乌斯常微分方程(ODE)热失控模型拟合到 ARC 数据的传统方法做出了一些假设,从而降低了所获模型的保真度和通用性。本文训练了化学反应神经网络 (CRNN),以拟合 N 方程阿伦尼乌斯 ODE 的动力学参数和从 Molicel 21700 P45B 获取的 ARC 数据。结果发现,这些模型能更好地逼近实验数据。通过对两方程和四方程模型的实验,证明了该方法的灵活性。利用所获得的动力学参数进行了三维热失控模拟,表明所获得的热失控模型适用于大规模模拟。本文章由计算机程序翻译,如有差异,请以英文原文为准。
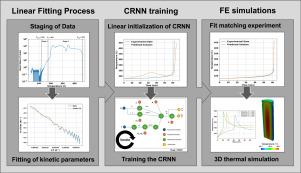
Chemical Reaction Neural Networks for fitting Accelerating Rate Calorimetry data
As the demand for lithium-ion batteries rapidly increases there is a need to design these cells in a safe manner to mitigate thermal runaway. Thermal runaway in batteries leads to an uncontrollable temperature rise and potentially battery fires, which is a major safety concern. Typically, when modeling the chemical kinetics of thermal runaway calorimetry data (e.g. Accelerating Rate Calorimetry (ARC)) is needed to determine the temperature-driven decomposition kinetics. Conventional methods of fitting Arrhenius Ordinary Differential Equation (ODE) thermal runaway models to ARC data make several assumptions that reduce the fidelity and generalizability of the obtained model. In this paper, Chemical Reaction Neural Networks (CRNNs) are trained to fit the kinetic parameters of N-equation Arrhenius ODEs to ARC data obtained from a Molicel 21700 P45B. The models are found to be better approximations of the experimental data. The flexibility of the method is demonstrated by experimenting with two-equation and four-equation models. Thermal runaway simulations are conducted in 3D using the obtained kinetic parameters, showing the applicability of the obtained thermal runaway models to large-scale simulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


