Cl 端接的 Si(100)-2 × 1 表面上的 NH3 氮化理论研究
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
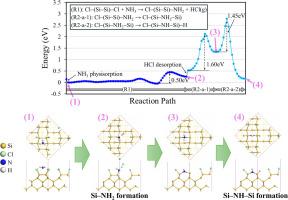
加强 SiN 沉积,特别是在原子层和化学气相沉积中使用 NH₃,对于提高硅基器件的性能至关重要。然而,虽然人们对 NH₃ 在清洁硅表面上的氮化作用有了很好的了解,但其在 Cl 端接硅表面上的行为在很大程度上仍未得到探索。本研究利用第一原理计算和热力学分析,研究了 NH3 在 Cl 端接的 Si(100)-2 × 1 表面上的氮化机理。NH3 与表面的 Si-Cl 发生反应,反应壁垒较低,生成 Si-NH2 和气态 HCl。随后,Si-NH2 通过 NH2 插入 Si-Si 二聚键和 H 迁移到 Si-dangling 键上形成 Si-NH-Si 结构。Si-NH-Si 在 Si-Si 二聚键上的形成比在 Si-Si 背键上更有利。热力学分析表明,NH3 氮化会导致形成 Si-NH-Si 结构,因为 Si-NH-Si 的形成比 Si-NH2 的形成在热力学上更稳定。此外,研究还证实,在较高温度和 NH3 分压下,Si-NH-Si 的形成反应更为有利。这些发现可用于改进氮化硅沉积工艺,提高硅基器件的性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Theoretical investigation of NH3 nitridation on Cl-terminated Si(100)-2 × 1 surfaces
Enhancing SiN deposition, particularly using NH₃ in atomic layer and chemical vapor deposition, is crucial for improving the performance of Si-based devices. However, while NH₃ nitridation on clean Si surfaces is well understood, its behavior on Cl-terminated Si surfaces remains largely unexplored. In this study, the mechanism of NH3 nitridation on Cl-terminated Si(100)-2 × 1 surfaces is investigated using first-principles calculations and thermodynamic analysis. NH3 reacts with Si–Cl on the surface with low reaction barriers, generating Si–NH2 and gaseous HCl. Subsequently, Si–NH2 forms a Si–NH–Si structure via NH2 insertion into the Si–Si dimer bond and H migration onto the Si-dangling bond. Si–NH–Si formation is more favorable on the Si–Si dimer bond than on the Si–Si back bond. Thermodynamic analyses indicate that NH3 nitridation leads to the Si–NH–Si structure, as Si–NH–Si formation is more thermodynamically stable than Si–NH2 formation. Moreover, it is confirmed that the Si–NH–Si formation reaction is more favorable at higher temperatures and NH3 partial pressures. These findings could potentially be used to improve SiN deposition processes and enhance the performance of Si-based devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


