锂-S-B 玻璃陶瓷:用于储能技术的新型电极材料
Q1 Materials Science
引用次数: 0
摘要
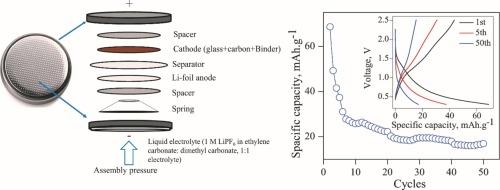
为可充电锂离子电池开发了电极硼酸锂基玻璃陶瓷(GC)的未来替代品。GC 的组成为 xNiO-(0.20-x)MnO2-0.80(Li2S:B2O3),其中 x 为 0.10、0.13、0.15 和 0.16。GC 是用熔淬技术制造的。利用 XRD 检测确定了 GC 的性质。扫描电子显微镜-电子显微镜分析表明了平板玻璃中成分的存在和分布。电池充放电测试表明,0.16NiO-0.04MnO2-0.8(Li2S:B2O3) (0.16Ni-0.04Mn) 玻璃陶瓷的电位范围为 0.8-1.1 V,第一个循环的放电容量为 70 mAh.g-1。此外,这些 GC 在超过 100 次循环中表现出卓越的循环稳定性。同时,电阻抗光谱(EIS)测量显示,0.16Ni-0.04Mn GC 在循环前后的锂扩散系数分别为 0.34 × 10-10 和 0.75 × 10-11 cm2.s-1,小于 0.10Ni-0.10Mn。基于同步辐射的 XANES 突出显示了 Ni2+ 的氧化态,以及 Mn2+/3+ 和 S-1 的混合态。在硼酸锂硫玻璃体系中添加镍和锰改善了其电化学特性,使其成为储能技术电极中一种非常有趣且经济可行的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Li-S-B Glass-Ceramics: A Novel electrode materials for energy storage technology
Future alternatives for an electrode lithium borate-based glass–ceramic (GC) has been developed for rechargeable lithium-ion batteries. The composition of the GC is xNiO-(0.20-x)MnO2-0.80(Li2S:B2O3), where x varies from 0.10, 0.13, 0.15, and 0.16. The GC were fabricated using the melt-quenching technique. The nature of the GC was determined using XRD examinations. The SEM-EDS analysis indicates the presence along with the distribution of components in the plate glasses. The battery charge/discharge tests showed that the 0.16NiO-0.04MnO2-0.8(Li2S:B2O3) (0.16Ni-0.04Mn) glass-ceramics exhibited a potential range of 0.8–1.1 V and a discharge capacity of 70 mAh.g−1 during the first cycle. Additionally, these GC demonstrated excellent cycling stability for over 100 cycles. As the same time, electrical impedance spectroscopy (EIS) measurements showed that the Li diffusion coefficient in 0.16Ni-0.04Mn GC was found to be 0.34 × 10−10 and 0.75 × 10−11 cm2.s−1 for before and after cycling, which is smaller than 0.10Ni-0.10Mn. Synchrotron-based XANES highlighted the oxidation state of Ni2+, as well as the mixing of Mn2+/3+ and S−1. The addition of Ni and Mn into the lithium-sulfur borate glass system has improved its electrochemical characteristics, making it a very interesting and economically viable option for energy storage technology electrodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Science for Energy Technologies
Materials Science-Materials Science (miscellaneous)
CiteScore
16.50
自引率
0.00%
发文量
41
审稿时长
39 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


