通过还原反应性胆固醇基嵌段共聚物和光热双亲化合物的共同组装构建的协同治疗纳米平台
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
癌症联合疗法(包括化疗-光疗)的目标是实现高效的抗肿瘤效果,同时最大限度地减少与传统化疗相关的不良反应。然而,提高非化疗策略在联合疗法中的贡献往往具有挑战性,因为这需要在单一给药系统中封装多种活性成分。然而,大多数常用的光热试剂难以大量装载,而且生物相容性较差。在此,我们通过一种共组装策略开发出了光热共胶束,该策略使用了一种胆固醇基液晶嵌段共聚物(LC-BCP)(LC 嵌段的侧链中含有二硫键)和一种含有胆固醇分子的巴豆双亲化合物(CBA)。这种方法可以将 CBA 有效地嵌入 LC-BCP 中,作为载药载体的功能成分。这些共胶束可以封装多柔比星(DOX),显示出可调的还原反应药物释放,并实现了近红外激光触发的光热疗法以及体内荧光和光热成像。激光照射后,共生微胞的光热活性可迅速诱导肿瘤细胞死亡并加速药物释放。体外和体内实验表明,这些药物载荷共胶束的协同光化学治疗效应为协同精准光热化学疗法提供了一条前景广阔的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
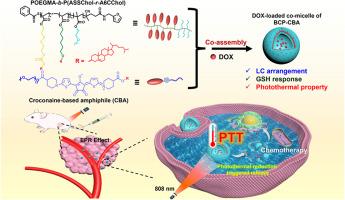
Nanoplatform for synergistic therapy constructed via the co-assembly of a reduction-responsive cholesterol-based block copolymer and a photothermal amphiphile
The goal of combination cancer therapy, including chemo-phototherapy, is to achieve highly efficient antitumor effects while minimizing the adverse reactions associated with conventional chemotherapy. Nevertheless, enhancing the contribution of non-chemotherapeutic strategies in combination therapy is often challenging because this requires multiple active ingredients to be encapsulated in a single delivery system. However, most commonly used photothermal reagents are challenging to be loaded in large quantities and have poor biocompatibility. Herein, we developed photothermal co-micelles through a co-assembly strategy using a cholesterol-based liquid crystal block copolymer (LC-BCP) with disulfide bonds in the side chain of the LC blocks and a croconaine-based amphiphile (CBA) containing a cholesterol moiety. This approach allowed the CBA to be effectively embedded within LC-BCPs, serving as the functional component of the drug-loaded carrier. These co-micelles could encapsulate doxorubicin (DOX), showed tunable reduction-responsive drug release, and enabled near-infrared laser-triggered photothermal therapy as well as in vivo fluorescence and photothermal imaging. Following laser irradiation, the photothermal activity of the co-micelles rapidly induced tumor cell death and accelerated drug release. In vitro and in vivo experiments demonstrated that the synergistic photo-chemotherapeutic effects of these drug-loaded co-micelles offer a promising avenue for synergistic precision photothermal-chemotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


