利用可注射水凝胶中的硫化氢引导免疫反应和破骨细胞生成平衡,治疗骨质疏松症
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
氧化应激水平升高、炎症和破骨细胞生成平衡失调经常成为骨质疏松症微环境的特征,阻碍了愈合和修复过程。现有的治疗方法范围有限,主要依赖于各种因素和药物。本研究介绍了一种可注射的水凝胶,其设计可响应 ROS 释放 H2S 气体。水凝胶的第一个网络由螯合了 S-aroylthiooxime (SATO) 和 H2S 生成基团的海藻酸钠(SA-SATO)组成,第二个网络由可光交联的 PEGDA 组成。通过整合与 ROS 反应的 Cys 释放微球,开发出了一种复合水凝胶,它具有良好的机械特性和生物安全性。体外实验证明,这种复合水凝胶能有效促进间充质干细胞的成骨分化,调节巨噬细胞极化,减少炎症反应,阻止细胞凋亡。此外,它还能释放 H2S 气体,缓解细胞中过量的 ROS。体内实验结果进一步验证了复合水凝胶在骨质疏松模型中促进骨缺损修复和再生的功效。总之,复合水凝胶通过协调成骨细胞和破骨细胞的活性、调节骨损伤的微环境和减少炎症,显示出作为解决骨质疏松性骨缺损的可行方法的潜力。因此,它是一种有效修复骨缺损的可行策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
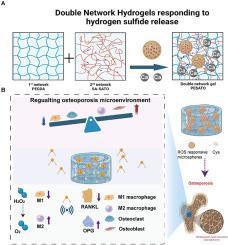
Harnessing hydrogen sulfide in injectable hydrogels that guide the immune response and osteoclastogenesis balance for osteoporosis treatment
Elevated levels of oxidative stress, inflammation, and a dysregulated osteoclastogenesis balance frequently characterize the microenvironment of osteoporosis, which impedes the processes of healing and repair. Existing treatment approaches are limited in scope and rely primarily on factors and drugs. An injectable hydrogel designed for the ROS-responsive release of H2S gas is presented in this study. The first network of the hydrogel comprises sodium alginate (SA-SATO) chelated with S-aroylthiooxime (SATO) and an H2S-generating group, while the second network is composed of photocrosslinkable PEGDA. Through the integration of Cys-releasing microspheres that are reactive with ROS, a composite hydrogel was developed that exhibited advantageous mechanical characteristics and biosafety. The composite hydrogel effectively promoted osteogenic differentiation of mesenchymal stem cells, modulated macrophage polarization, decreased inflammatory responses, and halted cell apoptosis, as evidenced by in vitro experiments. Additionally, it released H2S gas and mitigated excess ROS in cells. The efficacy of the composite hydrogel in promoting bone defect repair and regeneration in an osteoporotic model was further validated by in vivo findings. In summary, the composite hydrogel exhibits potential as a viable approach to address osteoporotic bone defects by harmonizing osteogenesis and osteoclast activity, modulating the microenvironment of bone injuries, and reducing inflammation. Consequently, it presents a viable strategy for the efficient repair of bone defects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


