生物正交益生菌平台在肿瘤深部时空释放纳米抗体,用于癌症化疗免疫疗法
IF 21.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
免疫检查点阻断疗法治疗实体瘤的有效性受到深部肿瘤内肿瘤免疫微环境激活有限的阻碍。免疫性细胞死亡为增强抗肿瘤免疫反应提供了一种前景广阔的方法。在癌症化学免疫疗法中整合这两种方法提出了一个新的视角。在此,我们开发了一种表达抗PD-L1纳米抗体的自矿化生物正交益生菌平台,用于癌症化疗免疫疗法。该平台可选择性地积聚并深入肿瘤组织,钯矿化益生菌在其中催化生物正交键裂解生成化疗药物,并诱导免疫性细胞死亡。在激光照射下,吲哚菁绿修饰的益生菌破裂并释放出纳米抗体,从而有效抑制肿瘤免疫逃避,并最终导致肿瘤特异性免疫反应。这一创新平台显著抑制了肿瘤生长和肺转移。总之,这些研究结果表明,通过生物正交性诱导免疫原性细胞死亡和 PD-L1 阻断作为一种治疗策略,协同增强抗肿瘤免疫,可以改善癌症化疗免疫疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
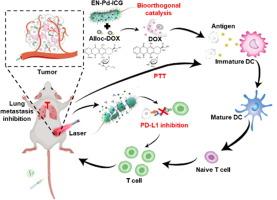
A bioorthogonal probiotic platform spatiotemporally releases nanobodies in deep tumor for cancer chemoimmunotherapy
The effectiveness of immune checkpoint blockade therapies in treating solid tumors is hindered by the limited activation of the tumor immune microenvironment within deep tumors. Immunogenic cell death offers a promising method to enhance anti-tumor immune responses. Integrating these two approaches in cancer chemoimmunotherapy presents a novel perspective. Here, a self-mineralized bioorthogonal probiotic platform with the expression of anti-PD-L1 nanobodies was developed for cancer chemoimmunotherapy. This platform selectively accumulated and deeply penetrated into tumor tissues, where the palladium-mineralized probiotics catalyzed bioorthogonal bond-cleavage to generate chemotherapeutic drugs and induced immunogenic cell death. Under laser irradiation, the indocyanine green modified probiotics were ruptured and released nanobodies which effectively suppressed tumor immune evasion and ultimately led to the tumor-specific immune responses. This innovative platform resulted in significant inhibition of tumor growth and lung metastasis. Overall, these findings suggest that synergistically enhancing antitumor immunity through the induction of immunogenic cell death via bioorthogonality and PD-L1 blocking as a therapeutic strategy could improve cancer chemoimmunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


