通过在聚合体和 Janus 纳米粒子团簇中的程序限制控制酶反应
IF 22
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在自然界中,区隔对于精确控制新陈代谢反应、与环境交换分子和信号以及细胞间通信至关重要。虽然人造细胞器和细胞为研究酶促反应提供了简化的条件,但对它们进行空间和方向控制仍是一项挑战。在这里,我们展示了自组织的催化纳米小室(CNCs),其中装有不同的酶,并特异性地附着在 Janus 纳米粒子(JNPs)上。这些簇通过程序化 DNA 杂交进行模块化组装。JNPs 的不对称具有独特的优势,它允许精确排列 CNCs,并以模块化的方式实现各种反应配置,包括单一、平行和级联酶反应。此外,集成了成像和治疗纳米组件的 JNP-CNCs 簇通过同时精确检测它们的体外位置和诱导细胞凋亡的活性氧(ROS)的产生,支持纳米otheranostic 应用。这种 JNP-CNCs 簇能够在纳米尺度上对酶反应进行空间和方向控制,在生物医学应用(包括蛋白质治疗和治疗学)方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
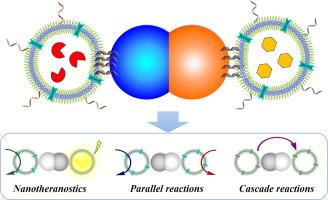
Controlled enzymatic reactions by programmed confinement in clusters of polymersomes and Janus nanoparticles
Compartmentalization is essential in nature for precisely controlling metabolic reactions, exchange of molecules and signals with the environment and inter-cell communication. While artificial organelles and cells offer simplified conditions for studying enzymatic reactions, it is still challenging to spatially and directionally control them. Here we present self-organized clusters combining catalytic nanocompartments (CNCs) loaded with different enzymes that are specifically attached to Janus nanoparticles (JNPs). The clusters are modularly assembled through programmed DNA hybridization. The asymmetry of the JNPs has unique advantages by allowing a precise arrangement of the CNCs and enabling, in a modular manner, various reaction configurations, including single, parallel and cascade enzymatic reactions. Additionally, JNP-CNCs clusters integrating imaging and therapeutic nanocompartments support nanotheranostic applications by simultaneous precise detection of their in vitro position and production of reactive oxygen species (ROS) that induce apoptosis. Such JNP-CNCs clusters provide both spatial and directional control of enzymatic reactions at the nanoscale and have high potential in biomedical applications, including protein therapy and theranostics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


