通过聚乙烯-亚胺原位功能化实现具有定制电荷性的晶体共价有机框架膜,从而实现高效药物排斥
IF 8.4
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
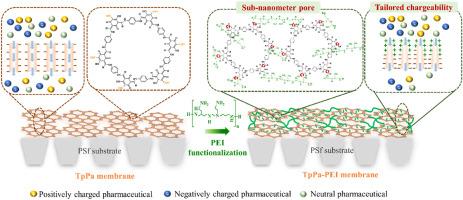
具有更好的亲水性、均匀的孔径分布和双电荷特性的纳滤(NF)膜非常适合改善药物排斥,尤其是中性和带正电荷的药物。在本文中,首先通过对甲苯磺酸(PTSA)介导的界面催化聚合(ICP)策略,使用 1,3,5-三异丙基氯葡萄糖醇(Tp)和对苯二胺(Pa)对 TpPa 膜进行原位结晶,然后用聚乙烯亚胺(PEI)进行后官能化,以缩小孔径、改善亲水性并定制膜电荷,从而提高药物排斥效果。PTSA 用作催化剂,以提高 TpPa 膜的结晶度。引入 PEI 后,孔半径从 0.382 ± 0.50 nm 缩小到 0.272 ± 0.33 nm,表面亲水性从 74.8° 提高到 37.0°,表面电荷从 -19.25 mV 转移到 11.15 mV。这种 PEI 功能化 TpPa(TpPa-PEI)层的两面都显示出异质电荷,顶部带正电,底部带负电。MgCl2 阻隔率从 13.7% 提高到 83.0%,而透水率却没有降低。此外,最佳 TpPa-PEI 膜的药物截留率和水渗透率分别比不使用 PTSA 制造的 TpPaIP-PEI 膜高出约 3.3 倍和 1.3 倍。此外,与原始 TpPa 膜相比,最佳 TpPa-PEI 膜的电正向性大大增强,使带正电荷的药物排斥率提高了 3-5 倍(普萘洛尔为 94.1%,舒必利为 97.2%,二甲双胍为 71.2%)。带负电荷的 TpPa-PEI 底层与缩小的孔径之间的协同作用使磺胺嘧啶的剔除率保持在 62.1%。机理研究进一步表明,PEI 渗入 TpPa 层 100 nm,并与醛基交联,从而定制了膜的电荷性、改善了亲水性并减小了孔径。通过 PEI 交联策略,可以合理地设计出亚纳米级的结晶 COF 层通道,具有精确定制的电荷性和亲水性,有望实现膜孔的功能化,并能显著提高水回用的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Crystalline covalent organic framework membrane with tailored chargeability for efficient pharmaceutical rejection by in-situ functionalization of polyethylene-imine
The nanofiltration (NF) membrane with better hydrophilicity, uniform pore size distribution, and dually charged properties is highly desirable to improve the pharmaceutical rejection, especially for the neutral and positively charged pharmaceuticals. Herein, a TpPa membrane was first in-situ crystallized via p-toluenesulfonic acid (PTSA)-mediated interfacial catalytic polymerization (ICP) strategy using 1,3,5-triformylphloroglucinol (Tp) and p-phenylenediamine (Pa), followed by the post-functionalization with polyethylene-imine (PEI) to narrow the pore size, improve hydrophilicity, and tailor membrane charges to enhance pharmaceutical rejection. PTSA was used as a catalyst to enhance the crystallinity of the TpPa membrane. The PEI introduction narrowed the pore radius from 0.382 ± 0.50 nm to 0.272 ± 0.33 nm, improved surface hydrophilicity from 74.8° to 37.0°, and shifted surface charge from −19.25 mV to 11.15 mV. This PEI-functionalized TpPa (TpPa-PEI) layers exhibited heterogeneous charges on both sides with a positively charged top and negatively charged bottom. MgCl2 rejection increased from 13.7 % to 83.0 % without sacrificing water permeance. Additionally, pharmaceutical rejection and the water permeance of the optimal TpPa-PEI membrane exceeded those of the TpPaIP-PEI membrane fabricated without PTSA by about 3.3 and 1.3 times, respectively. Furthermore, compared to the pristine TpPa membranes, the substantially enhanced electropositivity of the optimal TpPa-PEI membrane led to 3–5 times increase in positively charged pharmaceutical rejection (94.1 % for propranolol, 97.2 % for sulpiride, and 71.2 % for metformin). The synergy between the negatively charged TpPa-PEI bottom layers and the reduced pore size maintained a sulfadiazine rejection of 62.1 %. Mechanistic study further revealed that PEI penetrated 100 nm into the TpPa layer and cross-linked with the aldehyde groups, leading to tailored chargeability, improved hydrophilicity, and reduced pore size of the membranes. Via a PEI cross-linking strategy, sub-nanometer channels of crystalline COF layers can be rationally designed, featuring precisely tailored chargeability and hydrophilicity, promising functionalization of the membrane pores and remarkably robust for water reuse.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Membrane Science
工程技术-高分子科学
CiteScore
17.10
自引率
17.90%
发文量
1031
审稿时长
2.5 months
期刊介绍:
The Journal of Membrane Science is a publication that focuses on membrane systems and is aimed at academic and industrial chemists, chemical engineers, materials scientists, and membranologists. It publishes original research and reviews on various aspects of membrane transport, membrane formation/structure, fouling, module/process design, and processes/applications. The journal primarily focuses on the structure, function, and performance of non-biological membranes but also includes papers that relate to biological membranes. The Journal of Membrane Science publishes Full Text Papers, State-of-the-Art Reviews, Letters to the Editor, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


