离子水合在单价阴离子通过纳滤膜传输过程中的重要性
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
为了提高纳滤膜对特定溶质的选择性,需要从根本上了解这些膜的传输机制。在这项研究中,我们探讨了常见阴离子污染物(即硝酸盐和高氯酸盐)与常见氯离子相比的选择性趋势,并研究了这些趋势在松散聚酰胺纳滤膜中的基本传输机制。渗透实验表明,尽管硝酸盐和高氯酸盐是多原子且体积较大,但它们比单原子球形氯化物更快地渗透到膜中,这表明除了尺寸和电荷排斥外,还有其他机制在起着分离作用。与硝酸盐和高氯酸盐相比,氯化物的迁移焓障明显更高,这说明离子脱水在观察到的选择性中起着重要作用。为了进一步证实脱水的影响,我们在进料溶液中引入了各种疏水性不同的有机脂肪醇,从而系统地改变了离子的水合作用。脂肪醇的加入增强了硝酸盐和高氯酸盐的混沌特性,提高了它们的脱水能力,这反映在它们在酒精存在下的渗透性增强和焓障降低。我们还证明,由于硫酸盐对水结构的反作用,如果在系统中加入像硫酸盐这样的强各向同性阴离子,这种效应就会增强。最后,我们提出了阴离子在水-醇溶液中的行为机制,强调了离子水合在跨膜渗透中的关键重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
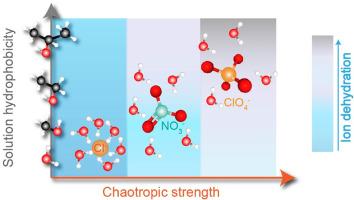
The importance of ionic hydration in the transport of monovalent anions through nanofiltration membranes
To improve the solute-specific selectivity of nanofiltration (NF) membranes, a fundamental understanding of the transport mechanisms in these membranes is required. In this study, we explored the selectivity trends of common anionic pollutants (i.e., nitrate and perchlorate) compared to the common chloride anion and examined the underlying transport mechanisms for these trends in loose polyamide NF membranes. Permeation experiments show that nitrate and perchlorate, despite being polyatomic and larger, permeate the membrane faster than the monoatomic, spherical chloride, suggesting that other mechanisms beyond size and charge exclusion govern the separation. Significantly higher enthalpic barriers measured for the transport of chloride compared to nitrate and perchlorate elucidated the important role of ion dehydration in the selectivity observed. To further support the influence of dehydration, we systematically altered the ions’ hydration by introducing various organic aliphatic alcohols of different hydrophobicity into the feed solution. The inclusion of aliphatic alcohols intensified the chaotropic characteristics of nitrate and perchlorate, augmenting their capacity to dehydrate, as reflected by their enhanced permeation and reduced enthalpic barrier in the presence of alcohols. We also demonstrated that this effect is boosted when a strong kosmotropic anion like sulfate is added to the system due to its counter effect on water structuring. We conclude with proposing mechanisms for the anion behavior in water-alcohol solutions that highlight the critical importance of ionic hydration in transmembrane permeation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Membrane Science
工程技术-高分子科学
CiteScore
17.10
自引率
17.90%
发文量
1031
审稿时长
2.5 months
期刊介绍:
The Journal of Membrane Science is a publication that focuses on membrane systems and is aimed at academic and industrial chemists, chemical engineers, materials scientists, and membranologists. It publishes original research and reviews on various aspects of membrane transport, membrane formation/structure, fouling, module/process design, and processes/applications. The journal primarily focuses on the structure, function, and performance of non-biological membranes but also includes papers that relate to biological membranes. The Journal of Membrane Science publishes Full Text Papers, State-of-the-Art Reviews, Letters to the Editor, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


