香草酸通过调节 PINK1/Parkin/Mfn2 信号通路减轻多柔比星诱导的心脏毒性
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
目的 本研究旨在探讨香草酸(VA)对多柔比星(DOX)诱导的心脏毒性的潜在保护作用,并揭示其潜在的分子机制。方法 通过腹腔注射多柔比星,建立 Sprague-Dawley (SD) 大鼠 DOX 诱导的心肌毒性模型。同时给予不同浓度的 VA 进行干预。建模后,使用超声波机对大鼠的心脏功能进行评估。使用生化分析仪检测大鼠血清中肌酸激酶同工酶 MB(CK-MB)的水平。用 HE 染色法观察心肌病理变化,用 Masson 三色染色法评估心肌纤维化程度。使用检测试剂盒观察心肌组织中超氧化物歧化酶(SOD)、丙二醛(MDA)和谷胱甘肽(GSH)的水平。用 Western 印迹和免疫组织化学方法检测 PINK1、Parkin、P62、LC3I/II、Mfn2、Drp1、OPA 和 Fis1 的蛋白表达水平。结果与 DOX 组相比,VA 的干预对多柔比星诱导的心脏毒性有改善作用,表现为大鼠左心室射血分数(LVEF)和左心室分数缩短(LVFS)的改善,以及左心室舒张末期直径(LVEDd)的缩小。此外,应用 VA 还降低了大鼠血清中 CK-MB 的水平。不过,在心率方面没有观察到有统计学意义的差异。组织学检查显示,在多柔比星诱导损伤的大鼠心脏中,施用 VA 会对心肌纤维的排列产生积极影响,并减轻心肌纤维化。此外,与 DOX 组相比,使用 VA 可减轻 DOX 诱导的心肌细胞凋亡。VA 还能上调 PINK1 和 Parkin 的表达水平,从而激活线粒体自噬。此外,VA 还能提高线粒体动力学蛋白 Mfn2、Drp1 和 Fis1 的表达水平,同时下调 OPA 的表达。结论 VA 通过上调 PINK1/Parkin/Mfn2 信号通路减轻了 DOX 诱导的心肌损伤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
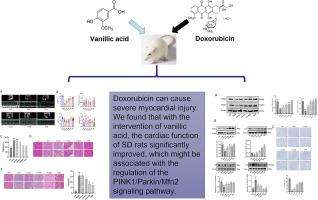
Vanillic acid attenuates doxorubicin-induced cardiotoxicity by regulating PINK1/Parkin/Mfn2 signaling pathway
Objectives
The aim of this study was to explore the potential protective effects of vanillic acid (VA) against doxorubicin (DOX)-induced cardiotoxicity and unravel the underlying molecular mechanisms involved.
Methods
The DOX -induced myocardial toxicity model was established in Sprague-Dawley (SD) rats by intraperitoneal injection of doxorubicin. Simultaneously, different concentrations of VA were administered for intervention. After modeling, rat cardiac function was evaluated using an ultrasound machine. The detection of creatine kinase isoenzyme MB (CK-MB) levels in rat serum using a biochemical analyzer. HE staining method was used to observe myocardial pathological changes, Masson's trichrome staining was performed to assess the degree of myocardial fibrosis. The observation of superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) levels in myocardial tissue using assay kits. Western blotting and immunohistochemistry was conducted to detect protein expression levels of PINK1, Parkin, P62, LC3I/II, Mfn2, Drp1, OPA, and Fis1.
Results
Compared to the DOX group, intervention with VA demonstrates ameliorative effects on doxorubicin-induced cardiotoxicity, as evidenced by improvements in left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS), and a reduction in left ventricular end-diastolic diameter (LVEDd) in rats. Additionally, the application of VA reduced the levels of CK-MB in rat serum. However, there were no statistically significant differences observed in heart rate. Histological examination reveals that VA administration positively impacts the arrangement of myocardial fibers and mitigates myocardial fibrosis in the rat hearts subjected to doxorubicin-induced injury. Moreover, relative to the DOX group, the use of VA alleviates DOX-induced myocardial cell apoptosis. VA treatment also upregulates the expression levels of PINK1 and Parkin, leading to the activation of mitochondrial autophagy. Furthermore, VA enhances the expression levels of mitochondrial dynamics proteins Mfn2, Drp1, and Fis1 while downregulating the expression of OPA. Among them, the high-dose group of VA (60 mg/kg·d) showed the most pronounced improvement in DOX-induced cardiotoxicity.
Conclusion
VA attenuated DOX-induced myocardial injury by up regulating the PINK1/Parkin/Mfn2 signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


