用于快速修复 MRSA 受损伤口和烧伤创面的氧化应激清除热激活 MXene 水凝胶
IF 21.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
由于细菌持续定植、高氧化应激和严重炎症,快速修复由多重耐药细菌感染或烧伤引起的复杂皮肤损伤仍是一项挑战。开发具有精确生物活性功能的高效生物材料是克服临床挑战的当务之急。在本研究中,我们介绍了一种基于 MXene(过渡金属碳化物和/或氮化物)的生物活性多功能水凝胶。这种水凝胶由 Ti3C2TX MXene 和聚(水杨酸)-Pluronic F127-聚(水杨酸)(FPSa@M)自组装而成,具有精确调节热抗氧化和抗炎环境的能力。FPSa@M 具有可注射性、快速凝胶化、导电性以及有益的抗氧化和光热作用。光热温度可调的 FPSa@M 水凝胶有效地实现了对高浓度多重耐药细菌的完全光热根除。此外,FPSa@M 水凝胶还能显著影响多种细胞行为,如刺激细胞增殖、清除活性氧、减少炎症因子表达、促进人脐静脉内皮细胞(HUVECs)迁移和 HUVECs 小管形成活性。在耐甲氧西林金黄色葡萄球菌(MRSA)感染或烧伤创面模型中,FPSa@M 能有效消除细菌感染,通过激活热休克蛋白 90 和血管生成,重塑创面愈合过程中的氧化应激和炎症微环境,从而显著促进创面修复。这项工作表明,热抗氧化活化生物材料在解决因多重耐药细菌感染或烧伤造成的大面积复杂组织缺损方面可能大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
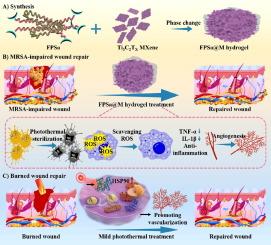
Oxidativestress-scavenging thermo-activated MXene hydrogel for rapid repair of MRSA impaired wounds and burn wounds
Rapid repair of complex skin injuries caused by multidrug-resistant bacterial infections or burn is still a challenge, due to the sustained bacterial colonization, high oxidative stress and severe inflammation. The development of efficient biomaterials strategy with precise bioactive functions is urgent in overcoming clinical challenge. In this study, we introduce a bioactive, multifunctional MXene (transition metal carbides and/or nitrides)-based hydrogel. This hydrogel, formed through the self-assembly of Ti3C2TX MXene and poly(salicylic acid)-Pluronic F127-poly(salicylic acid) (FPSa@M), exhibited the precise capabilities for regulating thermo-antioxidation and anti-inflammatory environments. FPSa@M exhibited the injectability, rapid gelation, electrical conductivity, and beneficial antioxidant and photothermal effects. The photothermal temperature-adjustable FPSa@M hydrogel effectively achieved complete photothermal eradication of high concentrations of multidrug-resistant bacteria. Additionally, FPSa@M hydrogel significantly impacted the multiple cellular behaviors, stimulating proliferation, scavenging reactive oxygen species (ROS), reducing inflammatory factor expression, promoting human umbilical vein endothelial cells (HUVECs) migration and tubule-forming activity of HUVECs. In the methicillin-resistant Staphylococcus aureus (MRSA)-infected or burn wound model, FPSa@M could efficiently eradicate bacterial infection, remodel the microenvironment of oxidative stress and inflammation in wound healing through activating the heat shock protein 90 and angiogenesis, thus significantly promote the wound repair. This work suggests that thermo-antioxidation activated biomaterials probably hold significant promise for addressing extensive complex tissue defects resulting from multidrug-resistant bacterial infections or burns.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


